Octreotide acetate dry powder inhalation preparation and preparation method thereof
A technology for inhalation of octreotide acetate and dry powder, which is applied in powder delivery, pharmaceutical formulations, peptide/protein components, etc. It can solve the problems of easy adhesion, aggregation, dispersion, etc., and achieve high-efficiency preparation, high emptying rate and deposition rate, and fluidity Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 Preparation and in vitro evaluation of octreotide acetate dry powder inhalation preparation
[0039] Mannitol is used as a drug carrier, ammonium carbonate is used as a porogen, the mass ratio of mannitol to ammonium carbonate is 8:2, the solid content is 1%, the drug content is 1%, and the spray drying solvent is water. Its preparation method comprises the following steps:
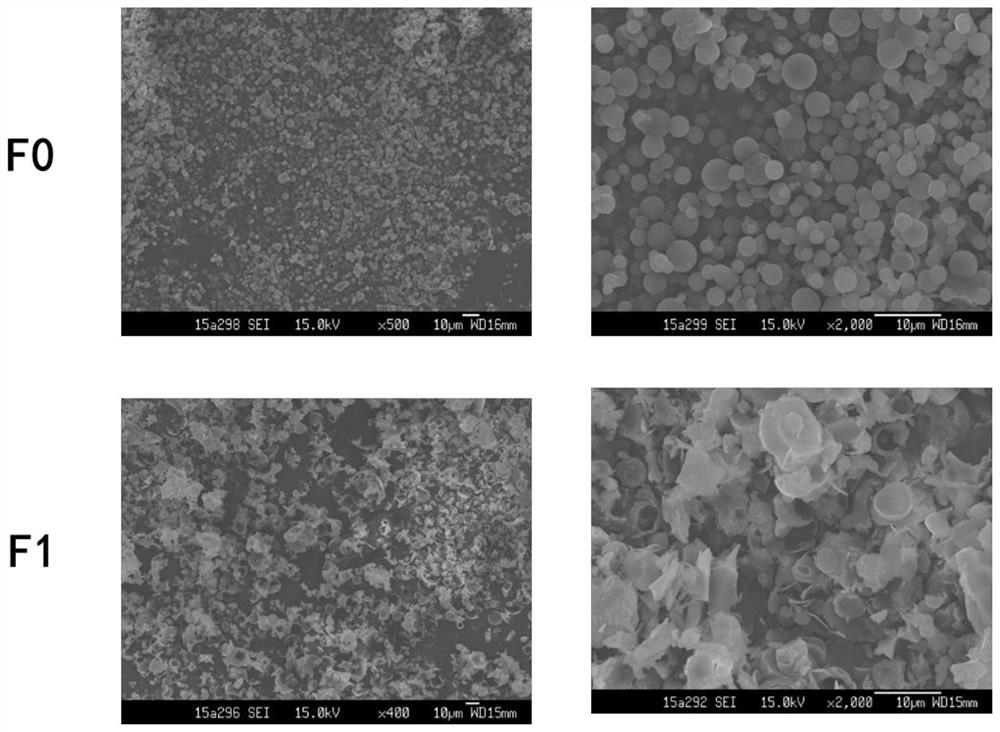
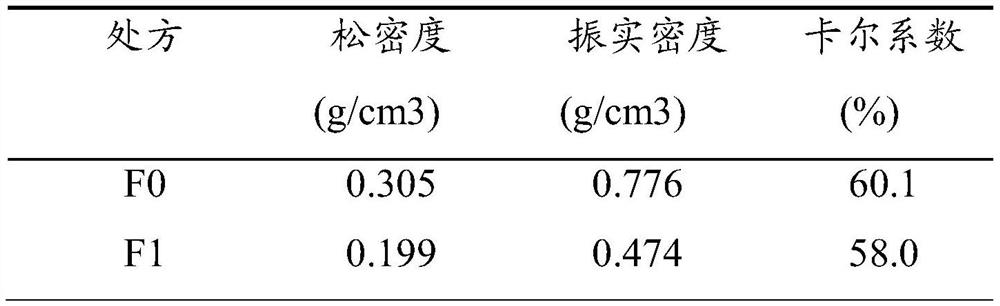
[0040]Weigh 0.8g of mannitol and 0.2g of ammonium carbonate, and dissolve them in 98ml of distilled water; weigh 1.0g of octreotide acetate, dissolve them in the above solution, and prepare a spray drying solution. During the spray drying process, water is used as the spray drying solvent, the inlet temperature (inlet air temperature) is 100°C, and the compressed air flow rate is 0.8m 3 / min, the atomization pressure is 150kPa, and the liquid supply rate is 1.5mL / min to obtain octreotide acetate fractal powder. The drug powder is packed into capsules and packed into a dry powder inhalat...
Embodiment 2
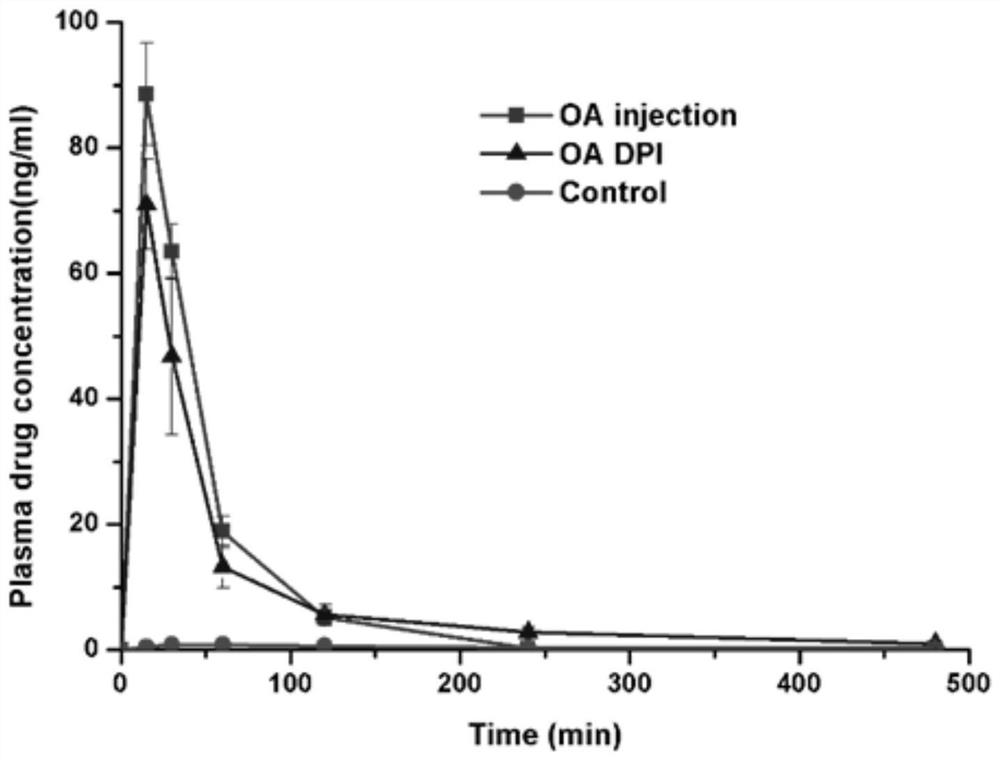
[0053] Example 2 Pharmacokinetic evaluation of octreotide acetate dry powder inhalation preparation
[0054] Mannitol is used as a drug carrier, ammonium carbonate is used as a porogen, the mass ratio of mannitol to ammonium carbonate is 1:1, the solid content is 3%, the drug content is 1%, and the spray drying solvent is water. Its preparation method comprises the following steps:
[0055] Weigh 1.5g of mannitol and 1.5g of ammonium carbonate, and dissolve them in 96ml of distilled water; weigh 1.0g of octreotide acetate, dissolve them in the above solution, and prepare a spray drying solution. During the spray drying process, water is used as the spray drying solvent, the inlet temperature is 110°C, and the air flow is 0.7m 3 / min, the atomization pressure is 165kPa, and the liquid supply rate is 1.8mL / min to obtain octreotide acetate fractal powder. The medicine powder is packed into a capsule and packed into a dry powder inhalation device to obtain the octreotide acetate...
Embodiment 3
[0063] With mannitol as the drug carrier and ammonium carbonate as the porogen, the mass ratio of mannitol:ammonium carbonate is 10:0, 9:1, 8:2, 7:3, 6:4, 5:5. Take 2.0g of mannitol and ammonium carbonate, dissolve in 97ml of distilled water; weigh 1.0g of octreotide acetate, and prepare a spray-dried solution according to the ratio of solid content 2% and drug content 1%. During the spray drying process, water is used as the spray drying solvent, the inlet temperature (inlet air temperature) is 120°C, and the compressed air flow rate is 0.6m 3 / min, the atomization pressure is 155kPa, and the liquid supply rate is 2.0mL / min to obtain octreotide acetate fractal powder. The drug powder is put into capsules and put into a dry powder inhalation device to obtain dry powder prescriptions of different ratios of mannitol and porogen ammonium carbonate.
[0064] The two parameters of powder yield and aerodynamic diameter were selected as the investigation index, and the results of ea...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com