Concentration gradient rhIL-2 dependent iNKT cell amplification method and application thereof
A technology of concentration gradient and cells, which is applied in the field of tumor cell immunotherapy, can solve the problems of low culture efficiency, achieve high amplification efficiency, enhance the immune enhancement of in vitro expanded iNKT cells and the effect of tumor immune monitoring
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1 Peripheral Blood Mononuclear Cells (PBMC) Extraction
[0040] (1) Take 20ml of anticoagulated venous blood in the morning.
[0041] (2) Add 15ml Lymphoprep separation solution into a 50ml centrifuge tube.
[0042] (3) Slowly add an equal volume of 0.9% sterile normal saline to the anticoagulated venous blood and gently invert three times to obtain diluted blood.
[0043] (4) Use a 2ml sterile dropper to slowly add the diluted blood to the surface layer of the Lymphoprep separation solution, and do not shake or turn it upside down after all is transferred.
[0044] (5) Balance the centrifuge tube, and centrifuge at room temperature for 30 minutes with a horizontal centrifuge (horizontal rotor) at 1700 rpm, ace / brake: 4 / 0.
[0045] (6) Aspirate excess plasma from the uppermost layer. Gently aspirate the lymphocyte layer with a 2ml sterile pipette, transfer to a new 50ml centrifuge tube, and aspirate the lymphocyte layer in all tubes into the same 50ml centrif...
Embodiment 2
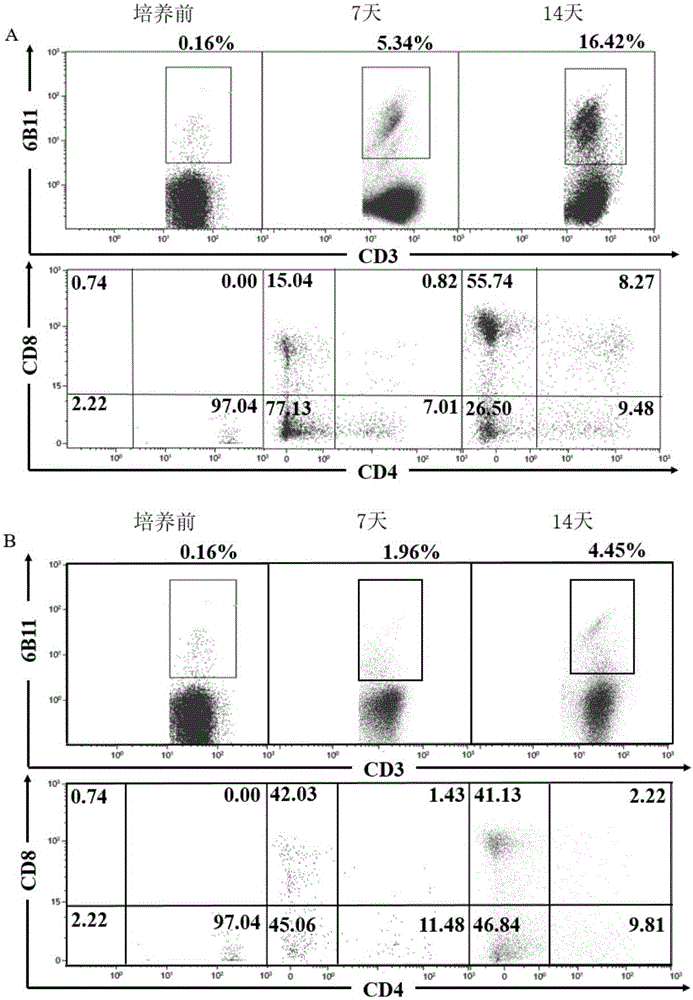
[0050] Example 2 Primary Expansion of iNKT Cells
[0051] (1) On the day of PBMC extraction, a final concentration of 100ng / ml α-GalCer was added and cultured for 48 hours.
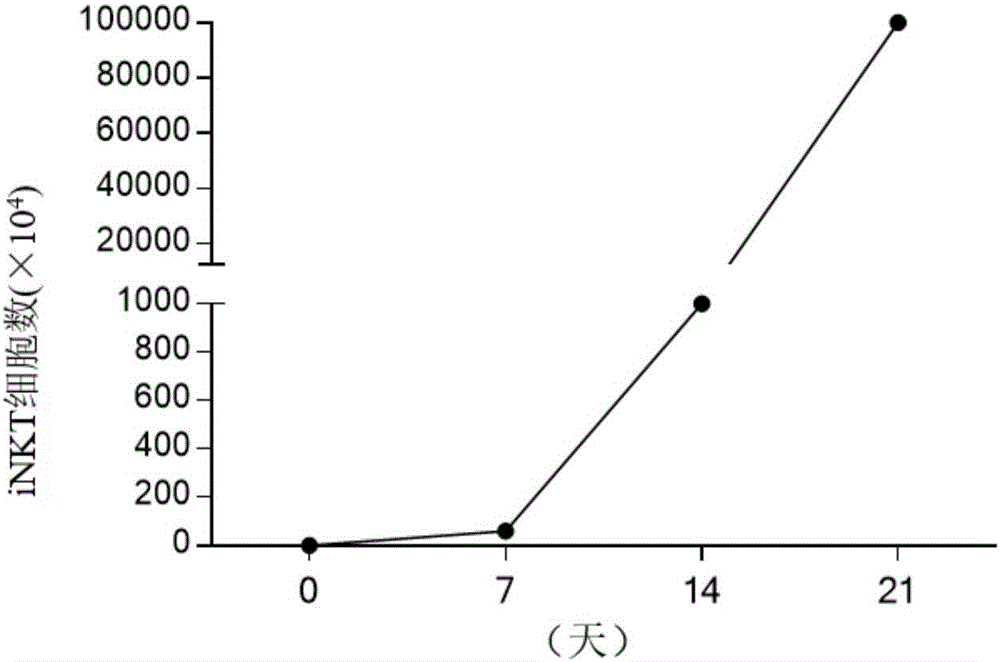
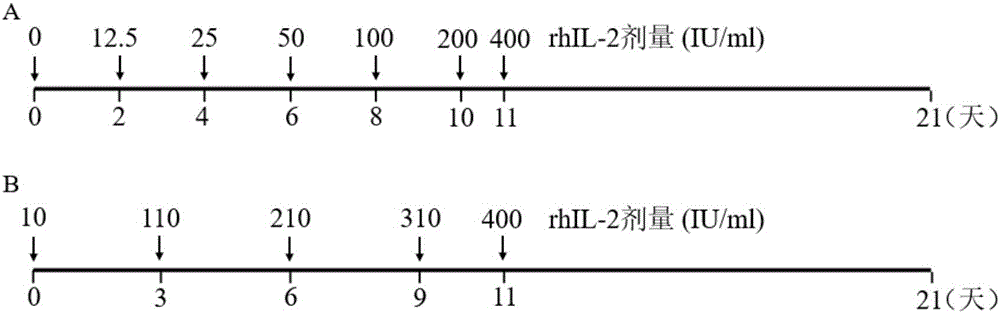
[0052] (2) The concentration of rhIL-2 was gradually increased in the culture medium, and the concentration of rhIL-2 increased in an equiproportional or equidifferential manner from the time the cells were isolated to the 11th day of culture.
[0053] (3) On the 11th day of culture, the concentration of rhIL-2 increased to 400IU / ml.
[0054] Depend on figure 1 As shown in A, the concentration of rhIL-2 increased in a proportional manner every 48 hours (except the 24-hour interval from the 10th day to the 11th day) from the time of cell isolation.
[0055] Depend on figure 1 As shown in B, the concentration of rhIL-2 increased in an arithmetic manner every 72 hours (except for the 48-hour interval from the 10th day to the 11th day) from the cell isolation.
Embodiment 3
[0056] Example 3 Induction and culture of dendritic cells
[0057] (1) Resuspend the PBMC obtained by density gradient centrifugation in AIM V medium, add it to a cell culture dish, and store at 37°C in 5% CO 2 to cultivate.
[0058] (2) After 3 hours, take out the culture dish, shake it gently, and suck out the suspended cells.
[0059] (3) Add AIM V medium containing 500IU / ml rhIL-4 and 500IU / ml rhGM-CSF to the culture dish.
[0060] (4) On the 3rd day and the 5th day after culture, the medium was changed in half, and fresh AIM V medium containing 500IU / ml rhIL-4 and 500IU / ml rhGM-CSF was added.
[0061] (5) Add rhTNF-α at a final concentration of 10ng / ml on the 6th day of culture.
[0062] (6) Mature dendritic cells were harvested on day 7.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com