Invariant natural killer T (iNKT) cell expressing targeted GPC3 chimeric antigen receptor and preparation and application for invariable natural killer T (iNKT) cell
A technology of chimeric antigen receptors and expression vectors, applied in the direction of receptor/cell surface antigen/cell surface determinant, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, genetically modified cells, etc. It can solve the problems that the preparation method and application of CAR-iNKT cannot be derived from it
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1 iNKT cell culture expansion
[0051] (1) Human venous blood was collected using lithium heparin anticoagulated vacuum blood collection tubes. Using Lymphoprep separation medium, human peripheral blood mononuclear cells (PBMCs) were separated by density gradient centrifugation.
[0052] (2) PBMCs were washed three times with AIM V cell culture medium, resuspended in AIM V cell culture medium containing a final concentration of 100ng / mlα-GalCer and 500IU / mlrhIL-2, and inoculated in a 75cm 2 Cell culture flask, transfer to 37 °C, 5% CO 2 , cultured in a cell incubator with saturated humidity.
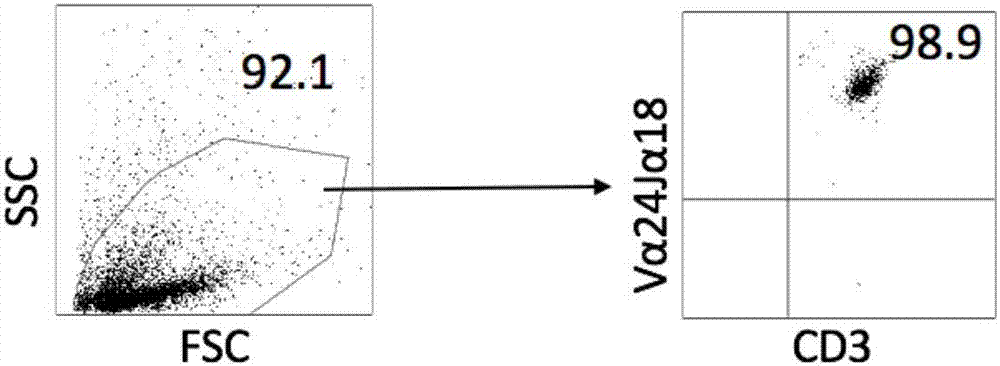
[0053] (3) Add AIM V cell culture medium containing 100ng / ml α-galactosylceramide and 500IU / mlrhIL-2 to the culture flask on the fourth day and the seventh day respectively, at 37°C, 5% CO 2 , continue culturing in a cell incubator with saturated humidity. Transfer cells to 225cm on day 10 2 Cell culture flasks, cultivated to the 14th day, use flow cytometry to sort ...
Embodiment 2
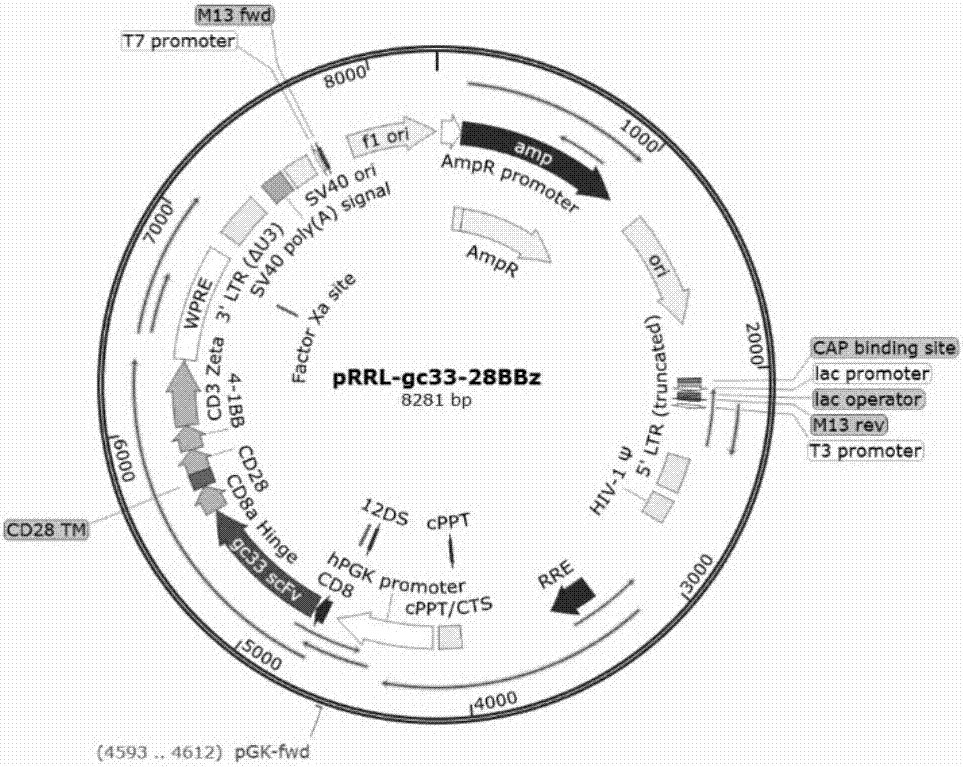
[0055] Example 2 Construction of chimeric antigen receptor protein lentiviral expression vector encoded by nucleic acid
[0056] Amplification and purification of nucleic acid fragments
[0057] (1) Amplification of scFv (GPC3) sequence
[0058] Sequence of template for scFv(GPC3) sequence:
[0059] GACGTGGTCATGACACAGAGCCCTCTGAGCCTGCCTGTGACACCTGGCGAACCTGCCAGCATCAGCTGTAGAAGCAGCCAGAGCCTGGTGCACAGCAACGGCAATACCTACCTGCACTGGTATCTGCAGAAGCCCGGCCAGTCTCCTCAGCTGCTGATCTACAAGGTGTCCAACCGGTTCAGCGGCGTGCCCGATAGATTTTCTGGCAGCGGCTCTGGCACCGACTTCACCCTGAAGATCTCCAGAGTGGAAGCCGAGGACGTGGGCGTGTACTACTGCAGCCAGAATACCCACGTGCCACCTACCTTTGGCCAGGGCACCAAGCTGGAAATCAAGAGAGGTGGCGGAGGATCTGGCGGAGGTGGAAGCGGCGGAGGCGGATCTCAAGTTCAGCTGGTTCAGTCTGGCGCCGAAGTGAAGAAACCTGGCGCCTCTGTGAAGGTGTCCTGCAAGGCCAGCGGCTACACCTTTACCGACTACGAGATGCACTGGGTCCGACAGGCTCCAGGACAAGGCTTGGAATGGATGGGAGCACTGGACCCCAAGACCGGCGATACAGCCTACAGCCAGAAATTCAAGGGCAGAGTGACCCTGACCGCCGACGAGTCTACAAGCACCGCCTACATGGAACTGAGCAGCCTGAGAAGCGAGGACCCGCCGTGTATTACTGTACCCGGTTCTACTCCTACA...
Embodiment 3
[0091] Example 3. Recombinant lentivirus infection of iNKT cells
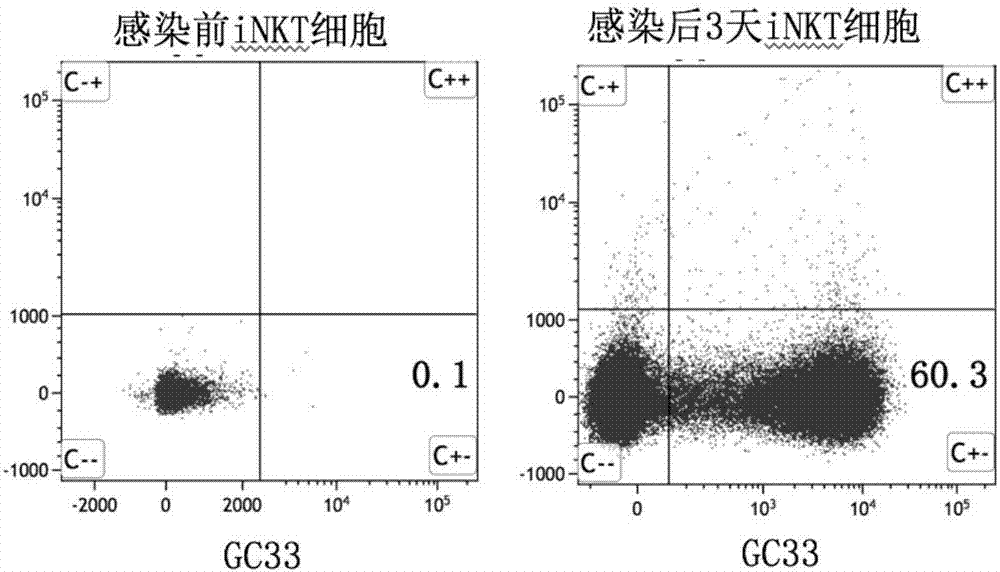
[0092] The iNKT cells obtained in Example 1 were approximately 1×10 6 Add AIM V culture medium at a density of 1:1, add magnetic beads coated with anti-CD3 and CD28 antibodies, and rhIL-2 at a final concentration of 500 IU / ml to stimulate culture for 24 hours. Then the iNKT cells were infected with the above recombinant lentivirus at MOI ≈ 5. Infected cells were harvested every other day with 5 × 10 5 / mL density for subculture, and at the same time, rhIL-2 with a final concentration of 500U / mL was added to the lymphocyte culture medium. Infected iNKT cells were detected by flow cytometry on day 8 of culture, and uninfected iNKT cells were used as a negative control. The positive results of iNKT cells infected with viruses expressing chimeric antigen receptors are shown in the attached image 3 shown. This result indicates that a certain positive rate of CAR-iNKT cells can be obtained by lentiviral infectio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com