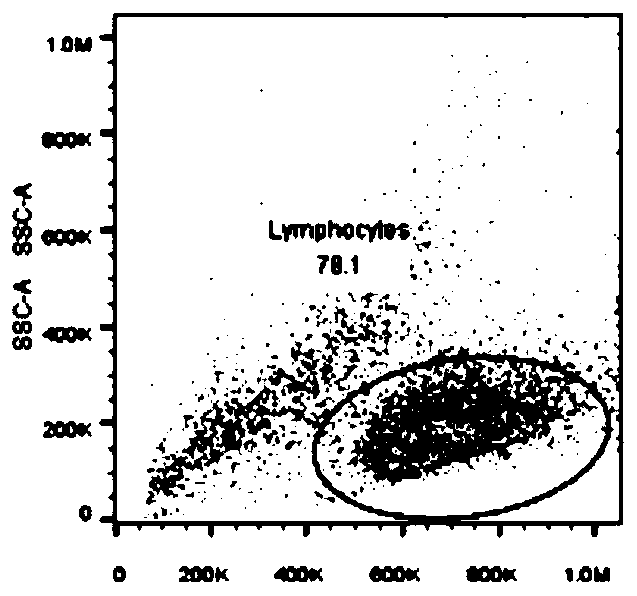
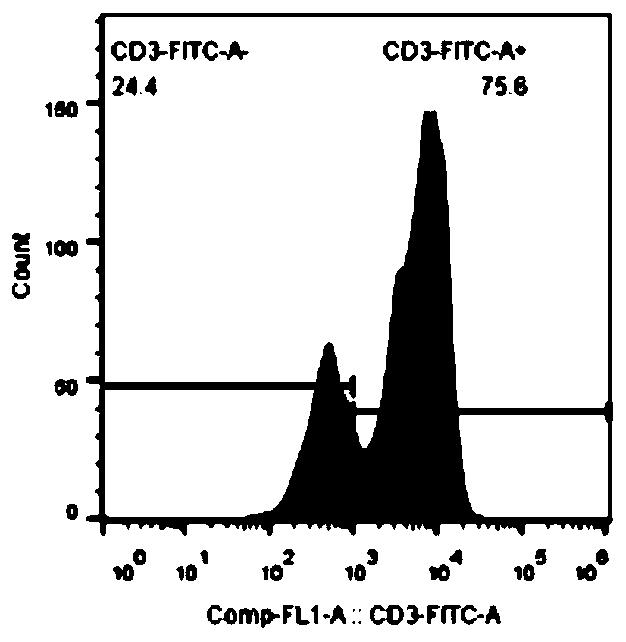
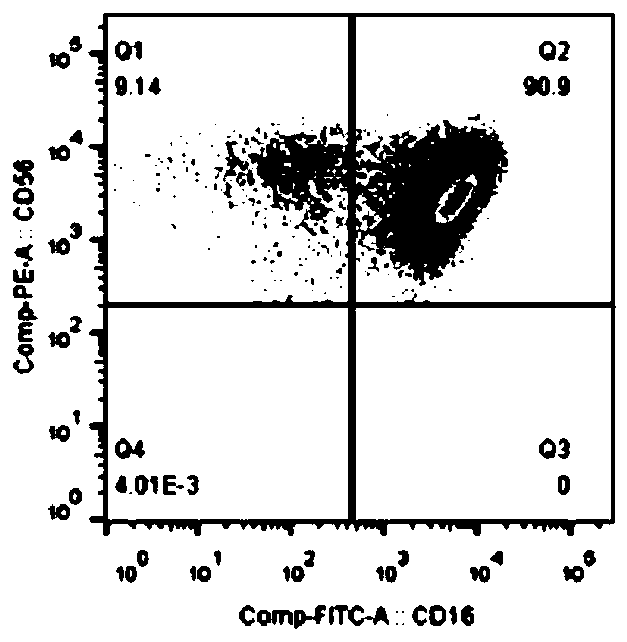
In-vitro amplification method of iNKT cells
An in vitro amplification and cell technology, applied in the field of cell expansion, can solve the problems of amplification multiple, purity and killing function, low ethical risk, etc., and achieve the effect of avoiding ethical risks, increasing amplification multiple, and improving killing performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] This embodiment is a method for extracting mononuclear cells in human peripheral blood. The extraction method includes the following steps:
[0047] (a) Take a sterile centrifuge tube, add 30ml of peripheral blood into the centrifuge tube, centrifuge at 2800rpm for 10min, and take the upper serum into the centrifuge tube, then put it in a water bath at 56°C for 30min, and then Centrifuge at 3500rpm for 20min, collect the supernatant into a clean centrifuge tube, store at 4°C for later use, and collect the lower layer of blood cells;
[0048](b) Add physiological saline to the blood cells to the whole blood volume, add hydroxyethyl starch to the blood cell solution at a volume ratio of 1:2 and mix well, settle for 30 minutes, take the supernatant into a centrifuge tube, and centrifuge at 2000rpm for 10 minutes , collect the precipitate and resuspend in 20ml saline;
[0049] (c) Slowly add the resuspended cell suspension into the supernatant in step (a) along the wall of...
Embodiment 2
[0051] Example 2 is a method for preparing DC cells, and the DC cells are prepared by the following method:
[0052] (a) mononuclear cells are obtained according to the method of Example 1;
[0053] (b) Add Corning88-551 medium to mononuclear cells and suspend them, adjust the inoculation density to 3×10 6 cells / ml, inoculate 10ml cell suspension into 90mm culture dish, at 37℃, 5% CO 2 Incubate for 4 hours in an incubator under certain conditions, then draw the supernatant into a blank centrifuge tube, and use PBS buffer to wash the culture bottle after absorbing the supernatant, and transfer the washing solution to a blank centrifuge tube containing the supernatant, Centrifugation and water washing, the centrifugation speed is 1500rpm, the time is 10min, and the water washing is washed twice with normal saline (the washing process should be light, so as not to wash down the monocytes);
[0054] (c) Add 10ml of Corning 88-551 blank medium to the washed centrifuge tube, and a...
Embodiment 3
[0057] This embodiment is a preparation method of iNKT cells, iNKT cells are prepared by the following method:
[0058] (a) Obtain mononuclear cells according to the method of Example 1, add Corning88-551 serum-free medium to the mononuclear cells and suspend, adjust the inoculation density to 3×10 6 cells / ml, inoculate 10ml cell suspension into 90mm culture dish, at 37℃, 5% CO 2 Incubate in an incubator under the conditions for 4 hours, then shake the culture dish slightly, draw the supernatant into a 50ml centrifuge tube, centrifuge at 2000prm for 10min, and collect the precipitated cells;
[0059] (b) Use Corning88-581 serum-free medium to resuspend and mix the pelleted cells, and the cell concentration is 2×10 6 cells / ml, then the cell suspension was transferred to the T-75 culture flask coated with CD3 (lug / ml) monoclonal antibody and CD16 (lug / ml) monoclonal antibody 5ml;
[0060] (c) Add IL-15, IL-2, IL-21 and α-galactosylceramide to the culture flask for cultivation,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com