Simple and effective method for induced amplification of iNKT cells and application
A NKT cell and cell technology, applied in animal cells, vertebrate cells, cell culture active agents, etc., can solve the problems of unsatisfactory anti-tumor effect, large demand for initial cells, and low remission rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0115] Example 1. Preparation of iNKT cells
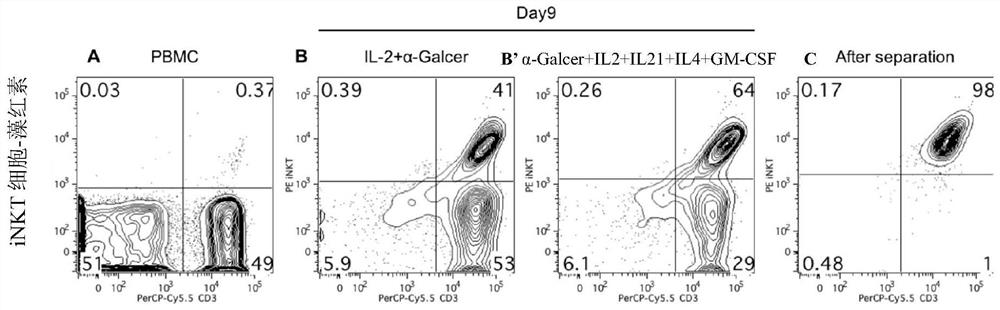
[0116]1) Separation of PBMCs: collect peripheral blood from the donor, dilute the whole blood with an equal volume of normal saline, add the lymphocyte separation solution and the diluted blood to the centrifuge tube at a ratio of 1:2, centrifuge at 2000rpm / min for 20 minutes, and collect the buffy coat cells , washed twice with normal saline, and centrifuged at 1500 rpm / min for 8 minutes to obtain PBMCs from peripheral blood mononuclear cells.
[0117] 2) Induce iNKT cells: resuspend PBMCs with lymphocyte medium, adjust the cell concentration to 2×10 6 / mL, add 100ng / mLα-Galcer, 50U / mL IL-2, 10ng / mL IL-21, 500U / mL IL-4 and 500U / mL GM-CSF, inoculate the cells in a 24-well plate and place at 37°C , 5% CO2 incubator. Observe the cell state every day, change the medium in half every other day, monitor the expansion effect by cell counting, and analyze the phenotype of iNKT cells by flow cytometry.
[0118] 3) Magnetic bead sortin...
Embodiment 2
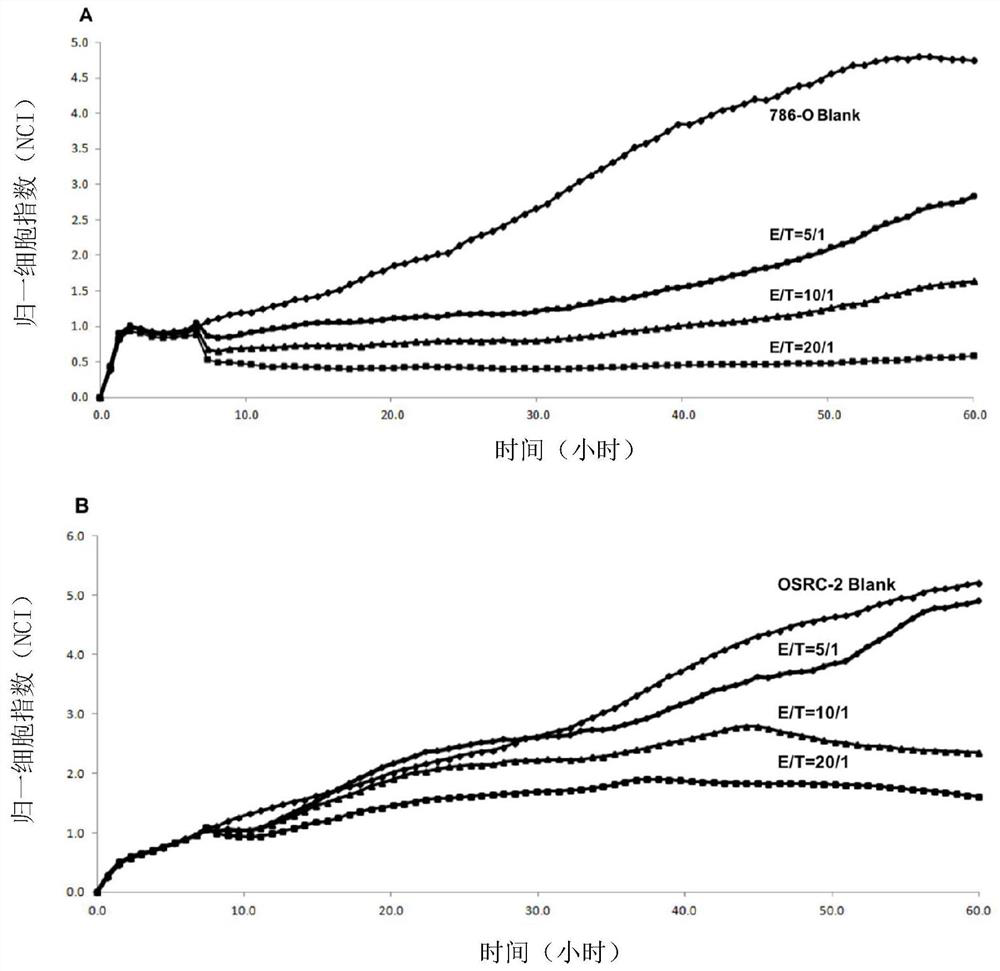
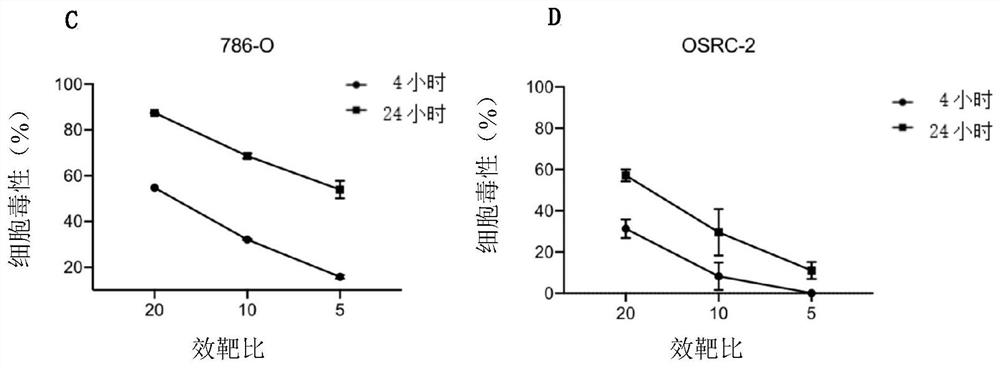
[0126] Example 2. The activity of iNKT cells to kill tumor cells in vitro
[0127] Effector cells: iNKT cells obtained in Example 1
[0128] Target cells: 786-O cells and OSRC-2 cells (this example takes human renal cancer cells as an example)
[0129] First, add 50 μL of tumor cell complete medium to the E-Plate detection plate of the xCELLigence cell function analyzer, and measure the background impedance value; collect the target cells in the logarithmic phase, adjust the concentration of the cell suspension to 1×105 / mL, and transfer to E -Add 100 μL of cell suspension to the plate, let it stand at room temperature for 30 minutes, and then place it on the detection platform; observe the real-time dynamic observation when the target cell proliferation is in the plateau stage, according to the effect-to-target ratio of 20:1, 10:1, 5:1 50 μL of effector cells were added, and only T cell culture medium was added to the control group; continue to observe the cell killing effe...
Embodiment 3
[0134] Example 3. Ability of iNKT cells to secrete cytokines
[0135] Digest tumor cells, wash and count to adjust the density to 1×10 5 / mL, pave a 24-well plate, and adhere to the wall overnight at 2 mL per well; collect the expanded iNKT cells, add them to the tumor cells at a ratio of 1:1 for co-cultivation, collect the supernatant after 24 hours, and detect IFN-γ and IL- 2. TNF-α.
[0136] image 3 The results showed that co-incubation of iNKT cells and renal cancer cells could secrete cytokines A.IFN-γ, B.IL-2 and C.TNF-α.
[0137] The above results prove that the iNKT cells obtained in Example 1 have the ability to secrete cytokines.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com