Drug composition containing sitagliptin and melbine
A technology for metformin and a composition is applied in the field of sustained-release preparations of sitagliptin and metformin, and achieves the effects of simple production process, consistent dissolution and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
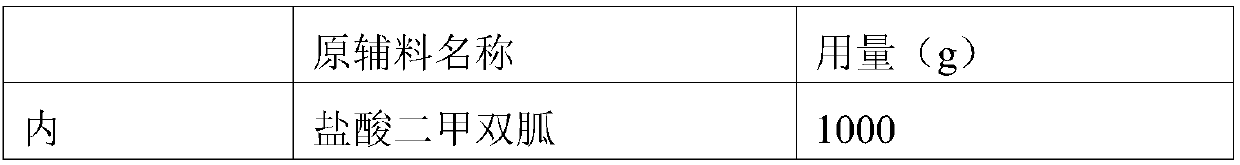
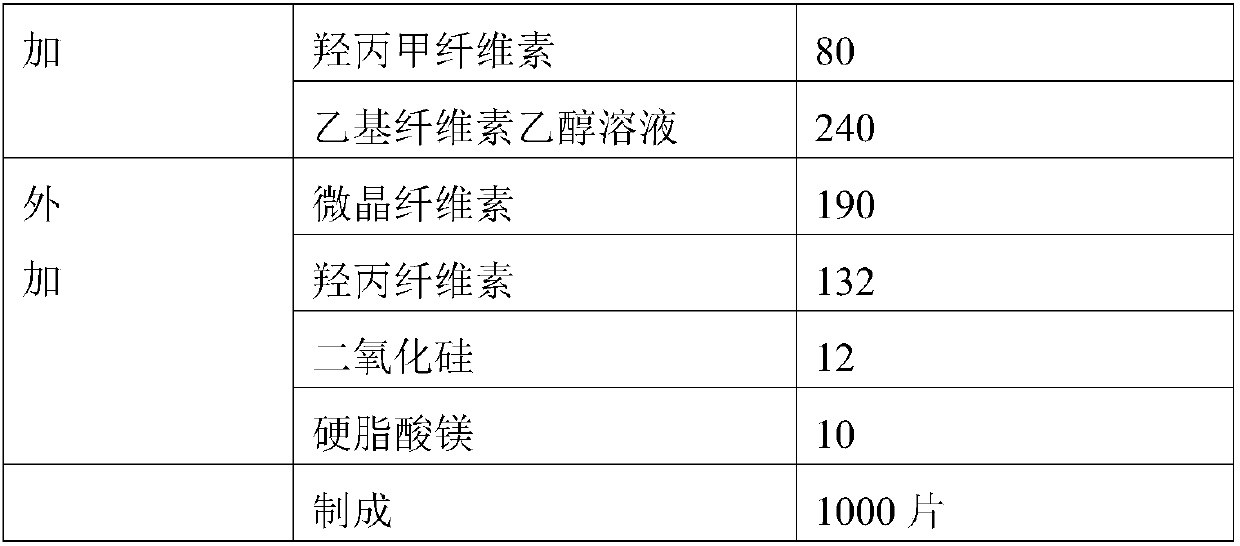
[0040] Embodiment 1: the preparation of metformin sustained-release tablet core
[0041] prescription:
[0042]
[0043]
[0044] Preparation:
[0045] Weigh the prescription amount of internally added raw and auxiliary materials, wet granulate with ethyl cellulose ethanol solution, dry in a fluidized bed, and granulate with a granulator to obtain internally added granules; mix the internally added granules with the prescription amount of externally added auxiliary materials, and measure After the content of the intermediate, the tablet is compressed to obtain the metformin hydrochloride sustained-release tablet core.
Embodiment 2
[0047] Sitagliptin suspension prescription:
[0048] effect
Name of raw material
Dosage (g)
Sitagliptin phosphate monohydrate (calculated as phosphate):
64.25
porogenic material
51.40
Film forming material
64.25
polyethylene glycol 4000
53.32
Antisticking agent
talcum powder
3.21
1927.50
production
1000 pieces
[0049] Preparation method: Dissolve polyethylene glycol 4000 in ethanol (95% concentration), add hydroxypropyl cellulose EF and stir to dissolve, mannitol, talcum powder, and sitagliptin pass through a 100-mesh sieve, and then add the above solution in sequence to obtain Sitagliptin suspension coating solution.
[0050] Use a high-efficiency coating machine, control the coating parameters as fan frequency 1000kw, air inlet temperature 80°C, material temperature 30°C, adjust t...
Embodiment 3
[0053] Sitagliptin suspension prescription:
[0054] effect
[0055] The method of evenly wrapping the sitagliptin suspension on the metformin tablet core prepared by the method of Example 1 is to use a high-efficiency coating machine to control the coating parameters as fan frequency 1200kw, inlet air temperature 70°C, material temperature 40°C, other preparation methods were the same as in Example 2, and 5000 coated tablets containing sitagliptin and metformin sustained-release layers were prepared.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com