Water-soluble drug sustained-release tablet and preparation method thereof
A water-soluble drug and slow-release tablet technology, applied in the field of medicine, can solve the problems of incomplete drug release, complex process, environmental pollution, etc., and achieve the effects of easy industrialization, simple preparation process, and environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Preparation Process:
[0037] Pre-mix captopril, HPMC-K4M, glyceryl behenate (Compritol 888 ATO), lactose and magnesium stearate (both passing through 80 mesh sieve) according to the doubling dilution method, and then pass through 80 mesh sieve After 3 times of thorough mixing, the powder is directly compressed into tablets, and each tablet contains 25 mg of captopril as sustained-release tablets.
Embodiment 2
[0039] components
[0040] Preparation process: with embodiment 1.
Embodiment 3
[0043] Preparation process: with embodiment 1.
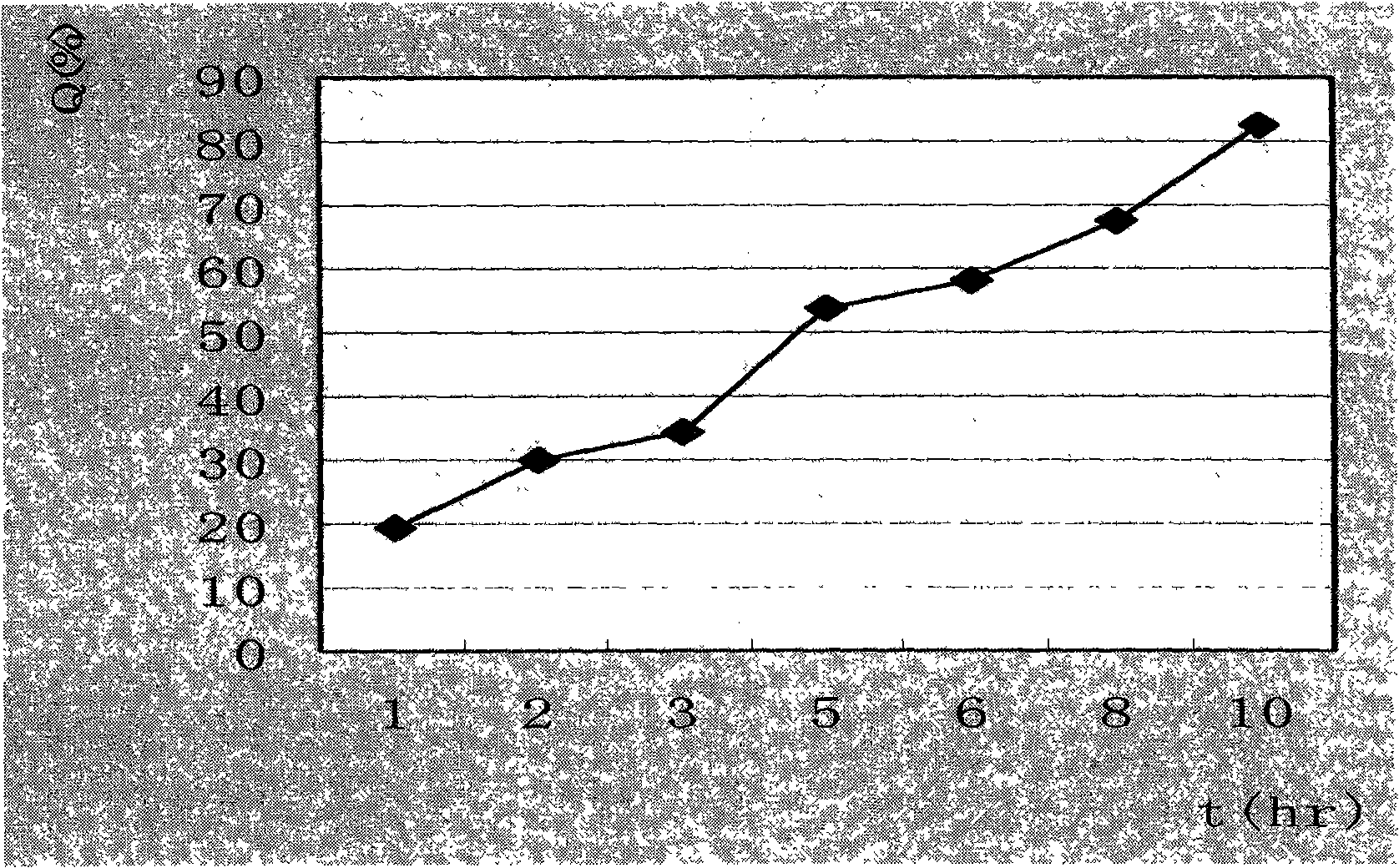
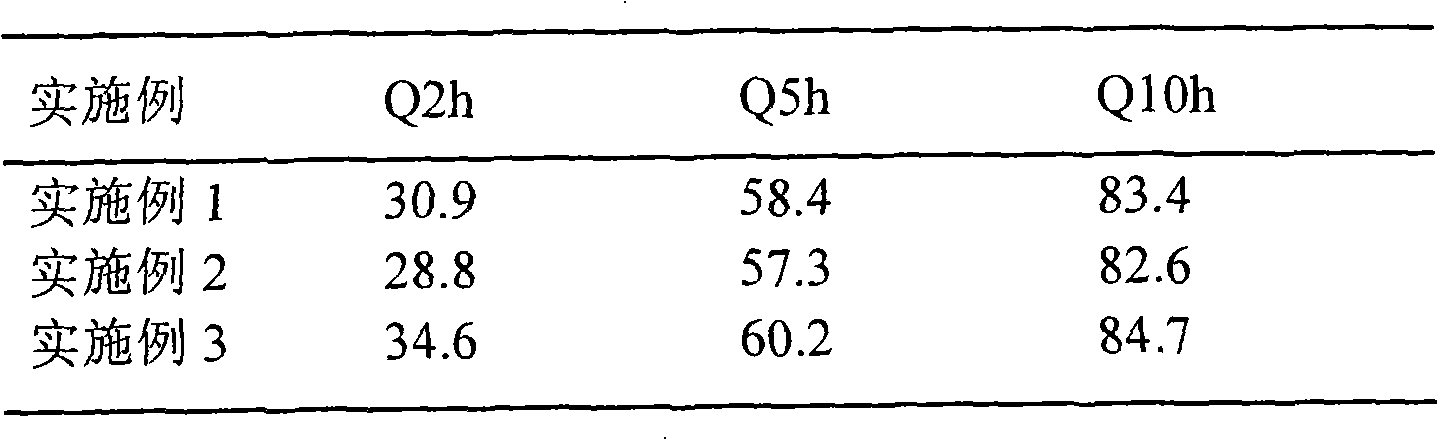
[0044] Dissolution test:
[0045] Carry out dissolution test to embodiment 1, embodiment 2 and embodiment 3 tablet, measure dissolution respectively in 2h, 5h and 10h, obtain corresponding cumulative release percentage (Q2h, Q5h, Q10h), the result is as follows:
[0046]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com