Aspirin sustained release tablet and preparing method thereof
A technology of aspirin and sustained-release tablets, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve problems such as intestinal mucosal irritation, reduce biodegradation, prolong curative effect, Steady and long-lasting effect of blood drug concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
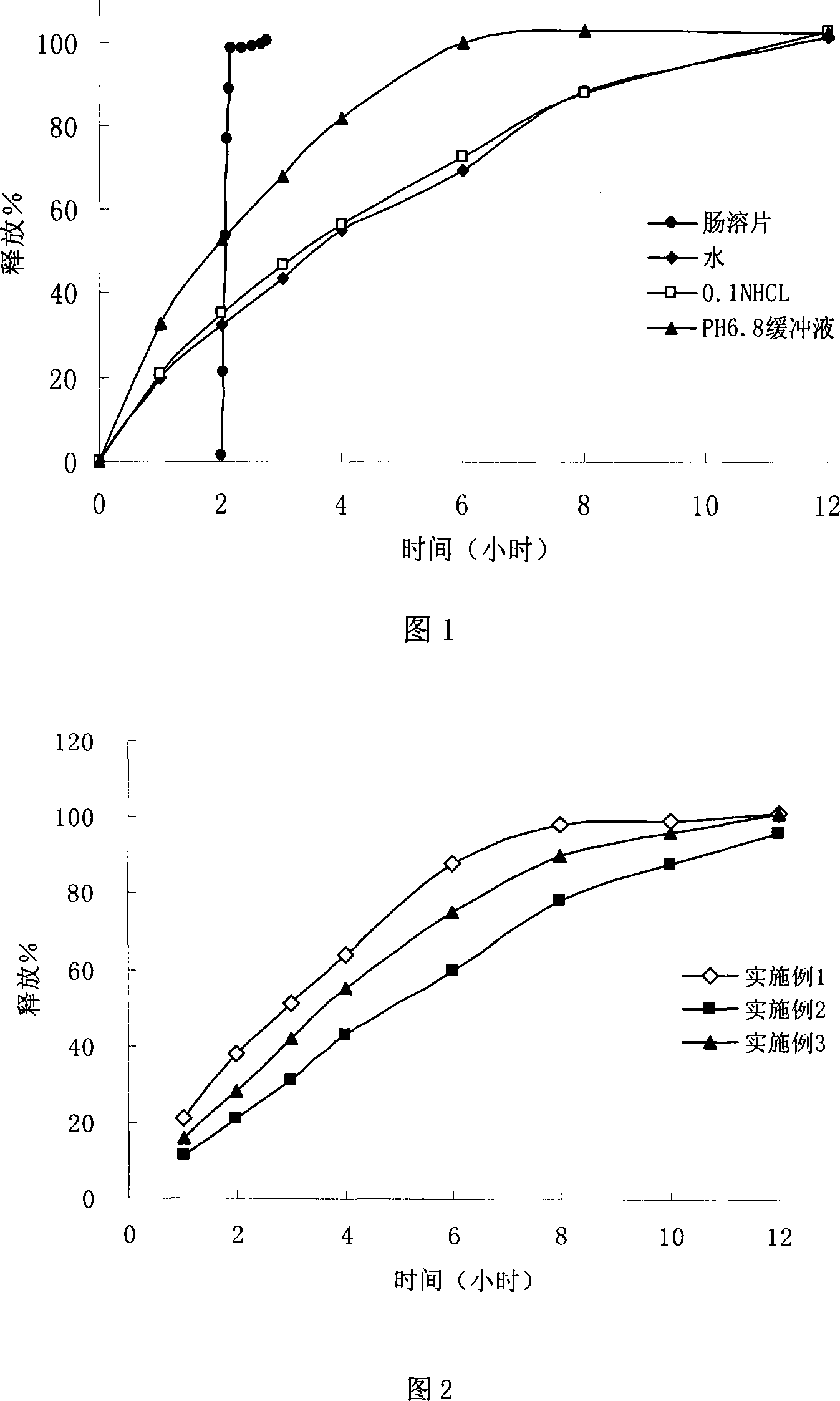
Image
Examples
Embodiment 1
[0042] The preparation prescription (1000) of aspirin slow-release tablet is as follows:
[0043] prescription
Dosage (1000 tablets)
aspirin
Ethyl cellulose 10CP
Polyacrylic resin No. II
75g
10g
7.5g
4g
[0044] tartaric acid
5% hypromellose alcohol solution
0.5g
20ml
2.7g
[0045] Preparation:
[0046]First prepare 5% hypromellose alcohol solution (50%), the method is as follows: get 50ml of medicinal alcohol, dilute to 95ml with distilled water, sprinkle 5g hypromellose on the 50% ethanol liquid surface, ultrasonically Minutes, moisten and mix, cover and place for 12 hours, mix well.
[0047] Take by weighing ethyl cellulose, polyacrylic resin No. II and starch (80 mesh) of prescription quantity respectively, mix at a slow speed for 5 minutes, then the principal ingredient aspirin (80 mesh) is mixed uniformly with the above-mentioned mixed powder...
Embodiment 2
[0049] The preparation prescription (1000) of aspirin slow-release tablet is as follows:
[0050] prescription
Dosage (1000 tablets)
aspirin
Ethyl cellulose 7CP
Eudragit L100-55
starch
5% hypromellose alcohol solution
Talc powder
75g
22.53g
10g
8g
0.32g
45ml
3.5g
[0051] Preparation:
[0052] First prepare 5% hypromellose alcohol solution (60%), the method is as follows: get 60ml of medicinal alcohol, dilute to 95ml with distilled water, sprinkle 5g hypromellose on the 60% ethanol liquid surface, ultrasonically 5 Minutes, moisten and mix, cover and place for 12 hours, mix well. Another prescribed amount of tartaric acid was dissolved with the prepared 5% hypromellose alcohol solution.
[0053] Weigh 8g of starch, 10g of Eudragit L100-55 and 22.53g of ethyl cellulose, stir and mix for 5 minutes, add the above-mentioned mixed powder and 75g of aspirin in the same am...
Embodiment 3
[0055] The preparation prescription (1000) of aspirin slow-release tablet is as follows:
[0056] prescription
Dosage (1000 tablets)
aspirin
Ethyl cellulose 7CP
Eudragit L100-55
starch
tartaric acid
5% hypromellose alcohol solution
Talc powder
75g
18g
7.5g
5g
0.48g
30ml
3.0g
[0057] Preparation:
[0058] The preparation method of 5% hypromellose alcohol solution is the same as that in Example 1, and the prescribed amount of tartaric acid is dissolved in the prepared 5% hypromellose alcohol solution.
[0059] Weigh 5g of starch, 7.5g of Eudragit L100-55 and 18g of ethyl cellulose, stir and mix for 5 minutes, add the mixed powder and 75g of aspirin in equal amounts, mix and stir evenly, and add the above-mentioned 5% hydroxypropyl cellulose under stirring Make a suitable soft material with 30ml of methyl cellulose alcohol solution, pass through a 20-mesh nylon sieve to make wet gr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com