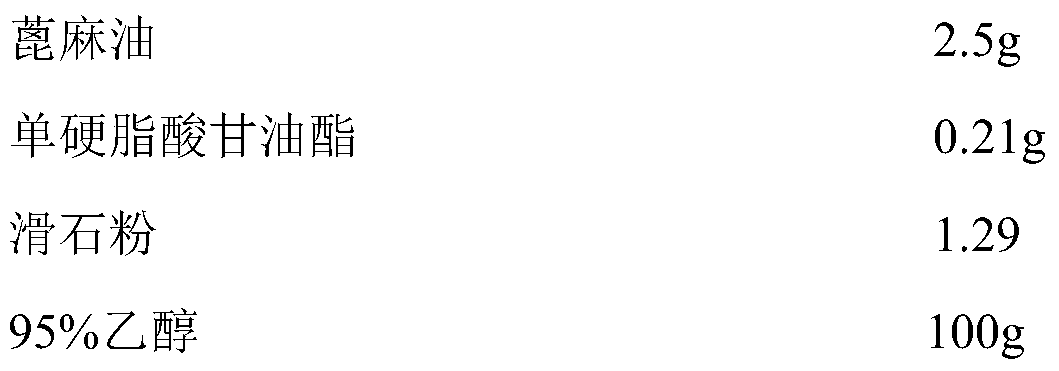A kind of aspirin enteric-coated tablet and preparation method thereof
A technology of aspirin and enteric-coated tablets, which is applied in anti-inflammatory agents, pharmaceutical formulas, non-central analgesics, etc. It can solve the problems of accelerated aspirin hydrolysis, free salicylic acid exceeding the standard, and greater impact on the content, so as to improve the release rate , easy industrial production, uniform color effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1 Preparation of aspirin enteric-coated tablets according to the present invention (specification 100mg / tablet, 1000 tablets)
[0025] Chip composition:
[0026]
[0027] Coating composition:
[0028]
[0029]
[0030] Preparation:
[0031] (1) Adhesive preparation: take 8 g of starch and 92 g of purified water to prepare 8% starch slurry, add disodium edetate, hypromellose acetate succinate, and ammonium oxalate, stir and dissolve, and use it as an adhesive;
[0032] (2) Granulation: Weigh aspirin and half of the prescribed amount of starch into the fluidized bed, pre-mix for 6 minutes, spray into the above-mentioned binder to granulate, dry until the moisture is 2.5%, add microcrystalline cellulose and the other half Recipe amount and dried starch at 100℃ for 24 hours, granulated with Φ1.5mm nylon screen;
[0033] (3) Tablet compression: add the granulated material into the mixer, mix for 5 minutes, and compress to obtain tablet cores. The tablet h...
Embodiment 2
[0036] Example 2 Preparation of aspirin enteric-coated tablets according to the present invention (specification 100mg / tablet, 1000 tablets)
[0037] Chip composition:
[0038]
[0039] Coating composition:
[0040]
[0041]
[0042] Preparation:
[0043] (1) Adhesive preparation: Take 7.6g of starch and 87.4g of purified water to prepare 8% starch slurry, add disodium edetate, hypromellose acetate succinate, and ammonium oxalate, stir and dissolve, and use it as an adhesive agent;
[0044](2) Granulation: Weigh aspirin and half of the prescribed amount of starch into the fluidized bed, pre-mix for 3 minutes, spray into the above-mentioned binder to granulate, dry until the moisture is 2.0%, add microcrystalline cellulose and the other half Recipe amount and dried starch at 100℃ for 24 hours, granulated with Φ1.5mm nylon screen;
[0045] (3) Tablet compression: add the granulated material into the mixer, mix for 2 minutes, and compress to obtain tablet cores. The ...
Embodiment 3
[0048] Example 3 Preparation of aspirin enteric-coated tablets according to the present invention (specification 100mg / tablet, 1000 tablets)
[0049] Chip composition:
[0050]
[0051] Coating composition:
[0052]
[0053] Preparation:
[0054] (1) Adhesive preparation: Take 8.4g starch and 96.6g purified water to prepare 8% starch slurry, add disodium edetate, hypromellose acetate succinate, ammonium oxalate, stir and dissolve, and use as adhesive agent;
[0055] (2) Granulation: Weigh aspirin and half of the prescribed amount of starch into the fluidized bed, pre-mix for 4 minutes, spray into the above-mentioned binder to granulate, dry until the moisture is 1.5%, add microcrystalline cellulose and the other half Recipe amount and dried starch at 100℃ for 24 hours, granulated with Φ1.5mm nylon screen;
[0056] (3) Tablet compression: add the granulated material into the mixer, mix for 4 minutes, and compress to obtain tablet cores. The tablet hardness is controll...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com