Aspirin enteric-coated tablet and preparation method thereof
A technology for aspirin and enteric-coated tablets, which can be applied to pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc. The effect of preventing water penetration and inhibiting hydrolysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0013] A preparation method for aspirin enteric-coated tablets, comprising the following steps:
[0014] S1, prepare aspirin tablet core;
[0015] In parts by weight, each part of the aspirin tablet core is a tablet core obtained by mixing 0.8-1.2 part of aspirin, 0.10-0.14 part of binder, 0.09-0.13 part of filler and 0.02-0.03 part of lubricant and directly compressing the tablet. Or the tablet core obtained by mixing 0.9-1.1 parts of aspirin, 0.11-0.13 parts of binder, 0.1-0.12 parts of filler and 0.023-0.029 parts of lubricant; or 1-1.05 parts of aspirin, 0.12-0.15 The tablet core obtained by directly compressing the mixture of 0.105-0.11 part of filler and 0.025-0.028 part of lubricant.
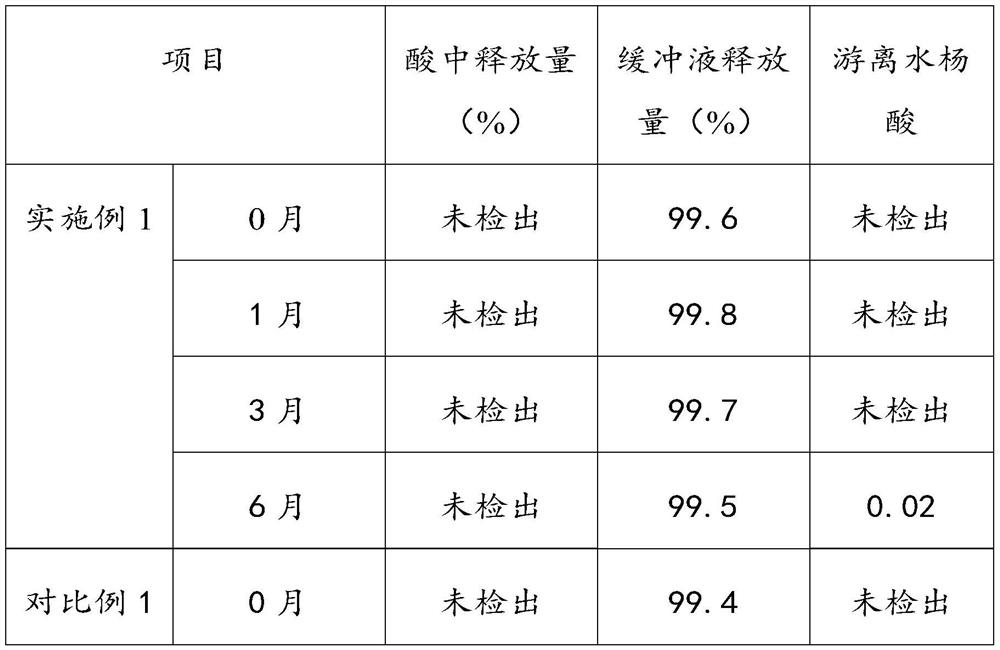
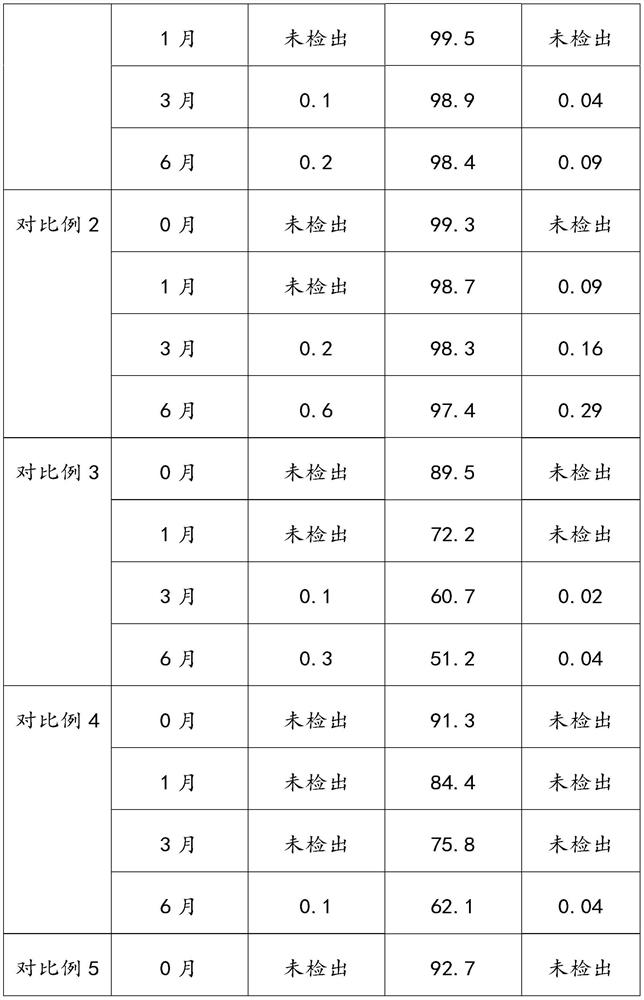
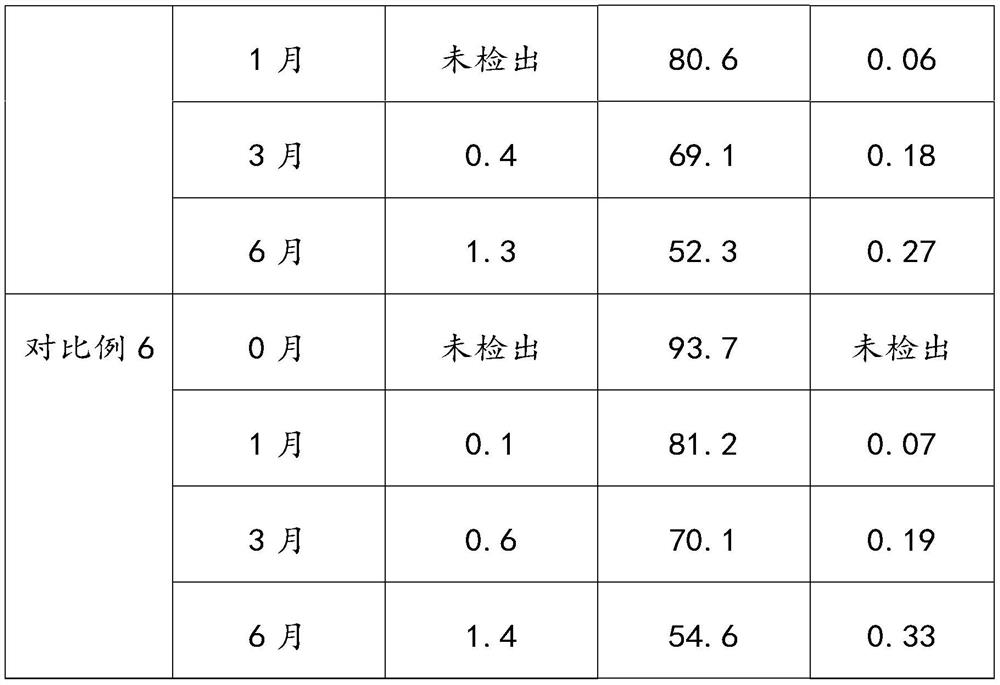
[0016] Aspirin is stable in dry air, and slowly hydrolyzes into salicylic acid and acetic acid when it encounters moisture. If aspirin is in contact with water, ethanol or other solvents, and then heated and dried under hot and humid conditions, it will promote the development of aspirin...
Embodiment 1
[0044] The present embodiment provides a kind of preparation method of aspirin enteric-coated tablet, comprises the following steps:
[0045] S1, prepare aspirin tablet core;
[0046] Prepare 560,000 aspirin enteric-coated tablets, mix 56Kg of aspirin, 5.8Kg of MCC-102, 6Kg of cornstarch and 1.4Kg of talcum powder and directly perform powder compression.
[0047] S2, preparing the first coating solution and the second coating solution;
[0048] S2.1, preparing the first coating solution;
[0049] Mix 2Kg methacrylic acid copolymer, 0.3Kg triethyl citrate, 1.0Kg talcum powder and 23Kg 95% ethanol to obtain the first coating solution. The methacrylic acid copolymer is a copolymer obtained by polymerization of methacrylic acid and acrylic acid thing.
[0050] S2.2, preparing the second coating solution;
[0051] Mix 2Kg of methacrylic acid copolymer, 0.15Kg of triethyl citrate, 0.3Kg of polysorbate 80, 1.0Kg of talc and 23Kg of 95% ethanol to obtain a second coating solution....
Embodiment 2-7
[0058] The preparation method of aspirin enteric-coated tablets provided in Examples 2-7 is basically the same as the preparation method of aspirin enteric-coated tablets provided in Example 1, except that the ratio of raw materials changes and the operating conditions are different.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com