Arginine aspirin tablet and preparation method thereof
A technology of arginine aspirin and arginine, which is applied in the field of arginine aspirin tablets and its preparation, can solve problems such as insufficient and complete reaction, influence on product stability, and lower production efficiency, so as to prolong the storage period and facilitate large-scale production. The effect of mass production and simple manufacturing process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
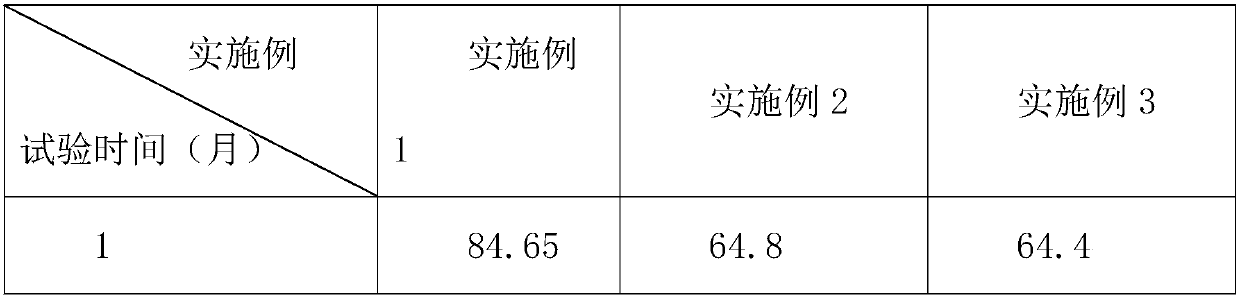
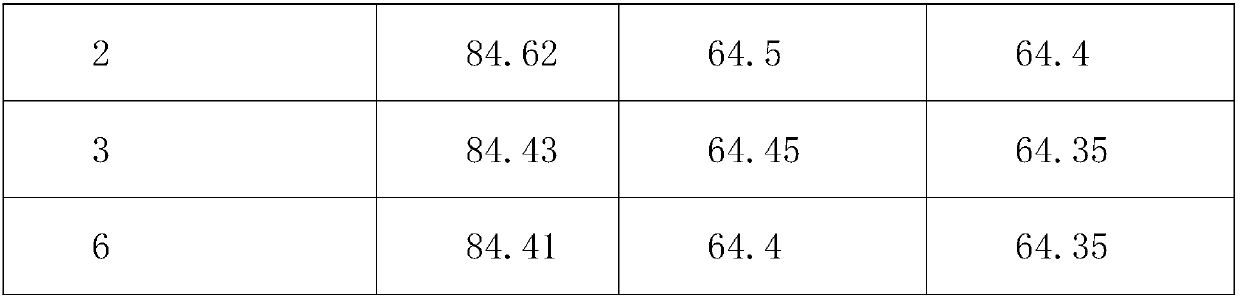
[0025] An arginine aspirin tablet is made from the following raw materials in the weight ratio: 85 parts of arginine, 85 parts of aspirin, 5 parts of starch slurry, 0.6 part of powdered sugar, 0.6 part of sago dextrin, 4 parts of pregelatinized starch 4 parts of calcium sulfate dihydrate, 3 parts of microcrystalline cellulose, 0.5 parts of tannic acid, 0.5 parts of magnesium stearate, 0.4 parts of sodium alginate, and 80 parts of absolute ethanol.
[0026] A preparation method for arginine aspirin tablet, comprising the steps of:
[0027] (1) Add the pregelatinized starch and magnesium stearate in the formula amount into deionized water, heat in a water bath at 75°C, and stir slowly while heating to obtain mixture A;
[0028] (2) Add the starch slurry, powdered sugar, and dextrin in the formula amount into deionized water, ultrasonically treat for 5 minutes, and heat in a water bath at 75°C to obtain mixture B;
[0029] (3) Take calcium sulfate dihydrate, microcrystalline cel...
Embodiment 2
[0034] An arginine aspirin tablet is made from the raw materials in the following weight ratio: 65 parts of arginine, 65 parts of aspirin, 3 parts of starch slurry, 0.3 part of powdered sugar, 0.3 part of sago dextrin, 2 parts of pregelatinized starch 2 parts of calcium sulfate dihydrate, 2 parts of microcrystalline cellulose, 0.3 parts of tannic acid, 0.3 parts of magnesium stearate, 0.3 parts of sodium alginate, and 60 parts of absolute ethanol.
[0035] A preparation method for arginine aspirin tablet, comprising the steps of:
[0036] (1) Add the pregelatinized starch and magnesium stearate in the formula amount into deionized water, heat in a water bath at 65°C, and stir slowly while heating to obtain mixture A;
[0037] (2) Add the starch slurry, powdered sugar and dextrin in the formula amount into deionized water, ultrasonically treat for 5 minutes, and heat in a water bath at 65°C to obtain mixture B;
[0038] (3) Take calcium sulfate dihydrate, microcrystalline cell...
Embodiment 3
[0043]An arginine aspirin tablet is made from the following raw materials in weight ratio: 65 parts of arginine, 65 parts of aspirin, 3 parts of starch slurry, 0.6 part of powdered sugar, 0.6 part of sago dextrin, 3 parts of pregelatinized starch 3 parts of calcium sulfate dihydrate, 3 parts of microcrystalline cellulose, 0.3 parts of tannic acid, 0.5 parts of magnesium stearate, 0.3 parts of sodium alginate, and 80 parts of absolute ethanol.
[0044] A preparation method for arginine aspirin tablet, comprising the steps of:
[0045] (1) Add the pregelatinized starch and magnesium stearate in the formula amount into deionized water, heat in a water bath at 65°C, and stir slowly while heating to obtain mixture A;
[0046] (2) Add the starch slurry, powdered sugar, and dextrin in the formula amount into deionized water, ultrasonically treat for 5 minutes, and heat in a water bath at 75°C to obtain mixture B;
[0047] (3) Take calcium sulfate dihydrate, microcrystalline cellulos...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com