Aluminum-magnesium-aspirin tablet (II)
A technology for aluminum-magnesium pirin tablet and aspirin, which is applied to the field of aluminum-magnesium pirin tablet and its preparation, and can solve the problems of unfavorable manufacturing cost, many processes, low β-cyclodextrin inclusion yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
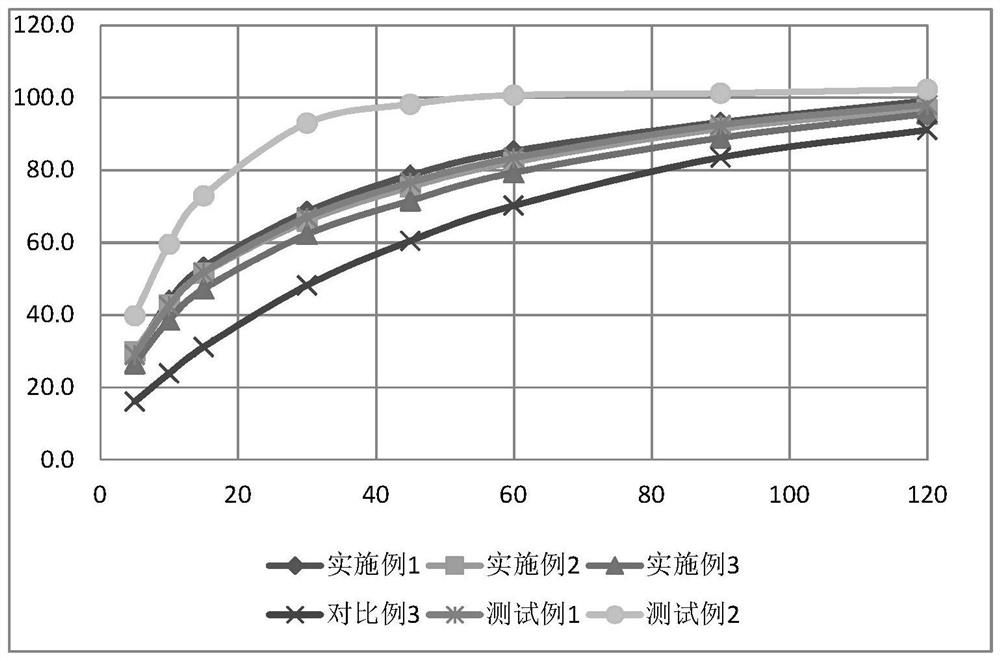
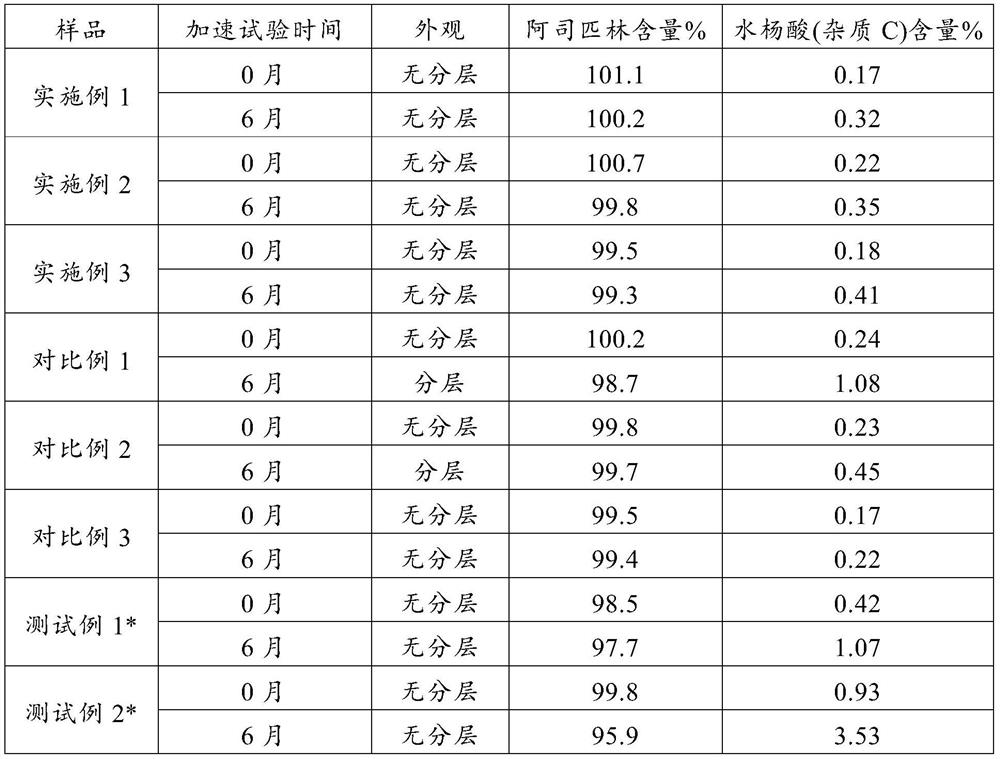
Image
Examples
Embodiment 1
[0054] A kind of aluminum-magnesium-pirin tablet (Ⅱ) of embodiment 1
[0055] The aluminum-magnesium-pirin tablet (II) described in this embodiment is a double-layer tablet composed of an aspirin layer and a buffer layer. In the aspirin layer, 80% w / w of aspirin is Coating with premixed coating agent.
[0056] The aluminum-magnesium-pirin tablet (II) of the present embodiment is prepared by the following method:
[0057] I. Preparation of Aspirin Layer Total Granules
[0058] (1) Preparation of aspirin-coated granules
[0059] Aspirin (40-150 mesh) 200 parts by weight
[0060] IR White 12 parts by weight
[0061] 48 parts by weight of purified water
[0062] Take the prescribed amount of water, add IR White, stirred and dissolved as aspirin powder coating solution. The aspirin powder is taken and placed in a fluidized bed bottom spray coating machine for coating until the coating liquid is completely coated to obtain aspirin coated granules. The operating parameter...
Embodiment 2
[0084] Embodiment 2 a kind of aluminum-magnesium-pirin tablet (Ⅱ)
[0085] The aluminum-magnesium-pirin tablet (II) described in this embodiment is a double-layer tablet composed of an aspirin layer and a buffer layer. In the aspirin layer, 80% w / w of aspirin is Coating with premixed coating agent.
[0086] The aluminum-magnesium-pirin tablet (II) of the present embodiment is prepared by the following method:
[0087] I. Preparation of Aspirin Layer Total Granules
[0088] (1) Preparation of aspirin-coated granules
[0089] Aspirin (40-150 mesh) 200 parts by weight
[0090] IR 10 parts by weight
[0091] 2.5 parts by weight of talcum powder
[0092] Titanium dioxide 2 parts by weight
[0093] Coloring agent 1 weight part
[0094] 83.5 parts by weight of purified water
[0095] Take the prescribed amount of water, add IR, talcum powder, titanium dioxide, coloring agent, stir and disperse evenly as aspirin powder coating solution. The aspirin powder is taken and pl...
Embodiment 3
[0109] Embodiment 3 a kind of aluminum-magnesium-pirin tablet (Ⅱ)
[0110] The aluminum-magnesium-pirin tablet (II) described in this embodiment is a double-layer tablet composed of an aspirin layer and a buffer layer. In the aspirin layer, 80% w / w of aspirin is Coating with premixed coating agent.
[0111] The aluminum-magnesium-pirin tablet (II) of the present embodiment is prepared by the following method:
[0112] I. Preparation of Aspirin Layer Total Granules
[0113] (1) Preparation of aspirin-coated granules
[0114] Aspirin (40-150 mesh) 200 parts by weight
[0115] Protect 10 parts by weight
[0116] Talcum powder 3 parts by weight
[0117] Coloring agent 1 weight part
[0118] 86 parts by weight of purified water
[0119] Take the prescribed amount of water, add Protect, talcum powder, and coloring agent are used as aspirin powder coating solution after stirring and dispersing evenly. The aspirin powder is taken and placed in a fluidized bed bottom spray...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com