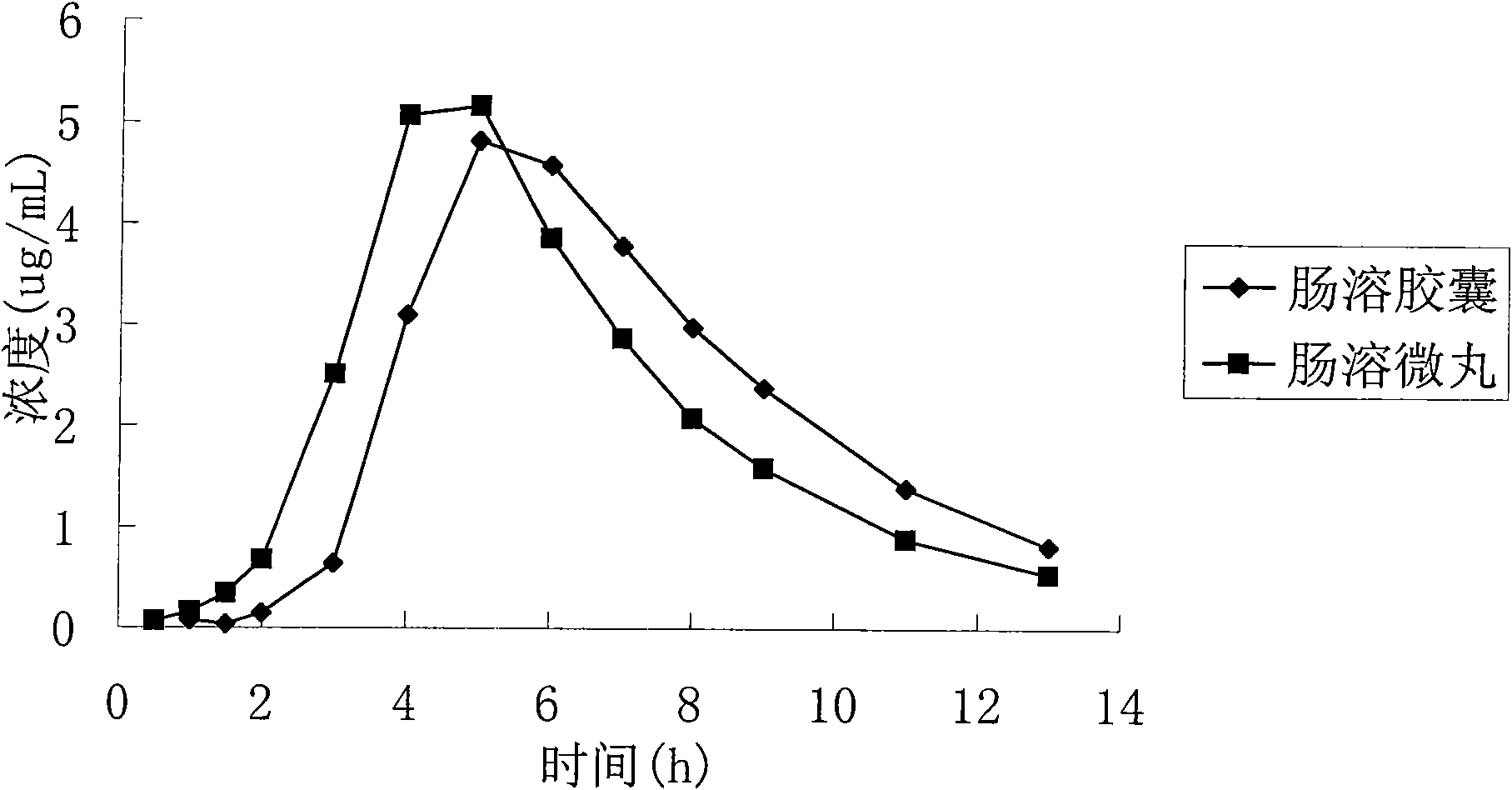
Aspirin enteric-coated pellet
A technology of aspirin and enteric-coated pellets, which can be used in medical preparations with non-active ingredients, blood diseases, and extracellular fluid diseases, etc. It can solve the problems that capsules are not easy to pass through the pylorus and the release time is unstable, so as to maintain blood drug concentration , the effect of maintaining balance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] A. Enteric-coated pellet preparation
[0047] Ingredients:
[0048] Aspirin 100g
[0049] Blank ball core 39.36g
[0050] Sodium carboxymethyl starch 3.2g
[0051] Low-substituted hydroxypropyl methylcellulose 4g
[0052] Isopropanol 160mL
[0053] Purified water 20mL
[0054] Eudragit L30D 37.3g
[0055] Glyceryl acetate 2.24g
[0056] Purified water 35mL
[0057] B. The method for preparing enteric-coated pellet preparation:
[0058] Dissolve low-substituted hydroxypropyl cellulose in isopropanol and purified water to form a binding agent; crush aspirin and sodium carboxymethyl starch; put the blank ball core in the coating pan, spray the binding agent, and then add the crushed After aspirin and sodium carboxymethyl starch until the operation is completed. Continue air drying for 3-4 hours to form drug-coated pellets.
[0059] Mix Eudragit L30D with glycerol acetate and purified water evenly to form an enteric coating solution; place the drug-coated pellets i...
Embodiment 2
[0062] A. Enteric-coated pellet preparation
[0063] Ingredients:
[0064] Aspirin 100g
[0065] Blank ball core 21.64g
[0066] Sodium carboxymethyl starch 2.8g
[0067] Low-substituted hydroxypropyl methylcellulose 3.8g
[0068] Ethanol 129mL
[0069] Purified water 18mL
[0070] Eudragit L30D 32.67g
[0071] Glyceryl acetate 1.96g
[0072] Purified water 27mL
[0073] B. Method of preparing the formulation:
[0074] Dissolve low-substituted hydroxypropyl cellulose in ethanol and purified water to form a binding agent; pulverize aspirin and sodium carboxymethyl starch; put the blank ball core in a fluidized bed coating pan, spray the binding agent, and then Add crushed aspirin and sodium starch glycolate until the operation is complete. Continue air drying for 3-4 hours to form drug-coated pellets.
[0075] Mix Eudragit L30D with glycerol acetate and purified water evenly to form an enteric coating solution; place the drug-coated pellets in a fluidized bed coating...
Embodiment 3
[0078] A. Enteric-coated pellet preparation
[0079] Ingredients:
[0080] Aspirin 100g
[0081] Blank ball core 35.08g
[0082] Sodium carboxymethyl starch 2.5g
[0083] Low-substituted hydroxypropyl methylcellulose 3.7g
[0084] Isopropanol 157mL
[0085] Purified water 20mL
[0086] Eudragit L30D 29.17g
[0087] Glyceryl acetate 1.75g
[0088] Purified water 25mL
[0089] B. Method of preparing the formulation:
[0090] Dissolve low-substituted hydroxypropyl cellulose in isopropanol and purified water to form a binding agent; crush aspirin and sodium carboxymethyl starch; put the blank ball core in the coating pan, spray the binding agent, and then add the crushed After aspirin and sodium carboxymethyl starch until the operation is completed. Continue air drying for 3-4 hours to form drug-coated pellets.
[0091] Mix Eudragit L30D with glycerol acetate and purified water evenly to form an enteric coating solution; place the drug-coated pellets in a coating pan, s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com