Lappaconitine gel patch and preparation method thereof
A technology of clathrate gel patch and high clathrate, which is applied in the directions of pharmaceutical formulations, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc. Poor performance, poor permeability and other problems, to achieve the effect of good compliance, reduce hydration, avoid allergies and irritation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
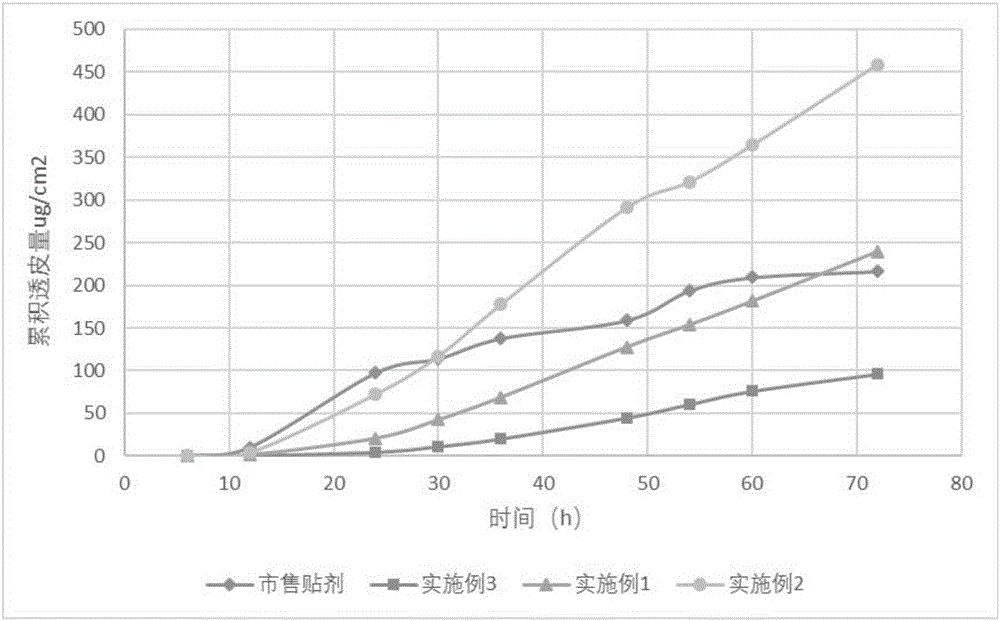
Image
Examples
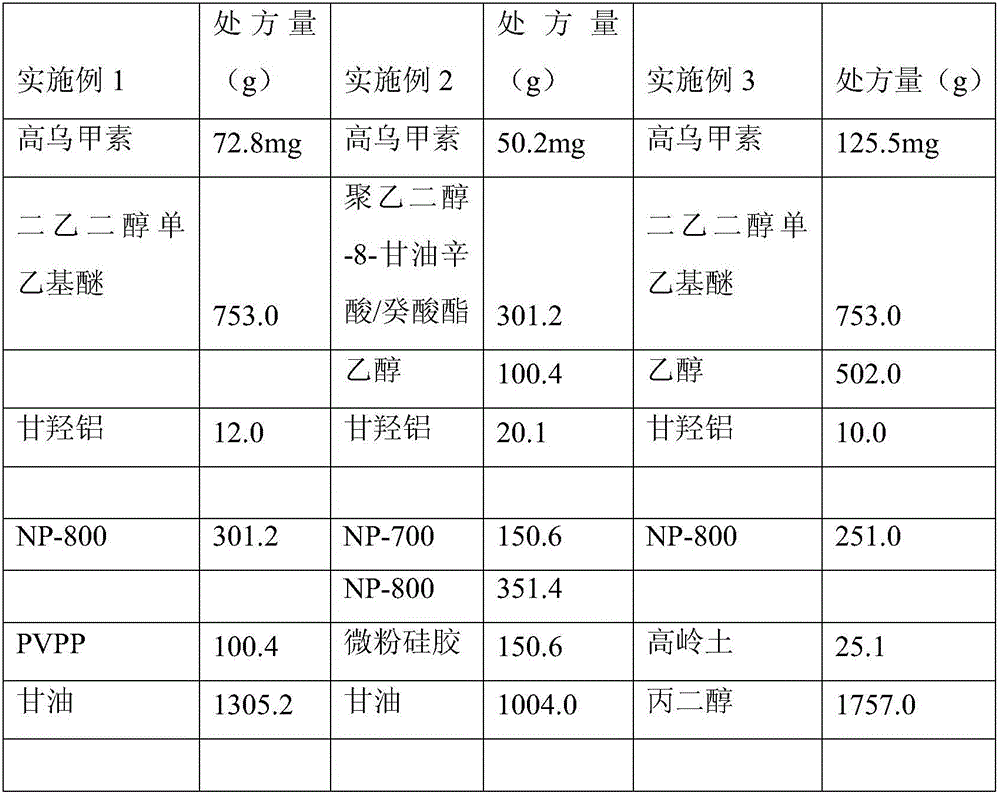
Embodiment 1
[0040] Preparation:
[0041] Weigh the prescribed amount of uricine raw material, add it to Transcutol P, stir and sonicate for 30 minutes to make a geranin suspension for later use; add glycolic aluminum to glycerin, stir well and then add Viscomate NP -800, stir evenly, then add the homogenate suspension, stir evenly, then add PVPP, stir evenly, as phase A. Add carbomer and tartaric acid to distilled water to dissolve, then add azone and Tween 80, stir evenly, and use it as phase B. Add phase B to phase A, stir quickly to an appropriate viscosity, apply it with a coating machine, cut it into a suitable size, and pack it in an aluminum foil bag to obtain a gel plaster.
Embodiment 2
[0043] Preparation:
[0044] Weigh the raw material of urine in the prescribed amount, add it to polyethylene glycol-8-glycerol caprylic acid / capric acid ester, ethanol, stir and sonicate for 30 minutes to make a suspension of urine, and set aside; Add aluminum hydroxy to glycerin, stir well, then add Viscomate NP-800 and NP-700, stir well, then add homogenate suspension, stir well, then add micropowder silica gel, stir well, as phase A. Separately take PVA and tartaric acid and add them into distilled water to dissolve them, then add oleic acid, azone and polyoxyethylene castor oil, and stir evenly to form phase B. Add phase B to phase A, stir quickly to an appropriate viscosity, apply it with a coating machine, cut it into a suitable size, and pack it in an aluminum foil bag to obtain a gel plaster.
Embodiment 3
[0046] Preparation:
[0047] Weigh the prescribed amount of urinine raw material, add it to Pharmasolve and ethanol, stir and sonicate for 30 minutes to make a suspension of urinine for later use; take aluminum glyoxate and add it to glycerin, stir well and then add Viscomate NP-800, stir evenly, then add gaolin suspension, stir well, then add kaolin, stir well, as phase A. Separately take PVP K-90 and tartaric acid and add them into distilled water to dissolve, then add eucalyptus oil and Tween 80, stir evenly, and use it as phase B. Add phase B to phase A, stir quickly to an appropriate viscosity, apply it with a coating machine, cut it into a suitable size, and pack it in an aluminum foil bag to obtain a gel plaster.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com