Pramipexole dihydrochloride transdermal patch and preparation method thereof
A technology of pramipexole hydrochloride and transdermal patch is applied to the transdermal patch of pramipexole hydrochloride and its preparation field, and can solve the problems of drugs entering the body and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The composition of the first layer (close to the release layer): 1.2g polyvinyl alcohol (PVA17-88), 0.3g polyvinylpyrrolidone (PVP), 0.2g eucalyptus oil, 0.12g azone, 800.2g Tween, Propylene glycol 0.4g, glycerin 0.2g, edetate disodium 0.1g, pramipexole hydrochloride 20mg.
[0028] The composition of the second layer (middle layer): polyvinyl alcohol (PVA17-88) 2.4g, polyvinylpyrrolidone (PVP) 0.6g, eucalyptus oil 0.4g, azone 0.24g, Tween 800.4g, propylene glycol 0.8g, glycerin 0.4g.
[0029] The composition of the third layer (close to the backing layer): polyvinyl alcohol (PVA17-88) 1.2g, polyvinylpyrrolidone (PVP) 0.3g, eucalyptus oil 0.2g, azone 0.12g, Tween 800.2g, propylene glycol 0.4 g, glycerin 0.2g, disodium 7-diamine tetraacetate 0.1g, pramipexole hydrochloride 60mg.
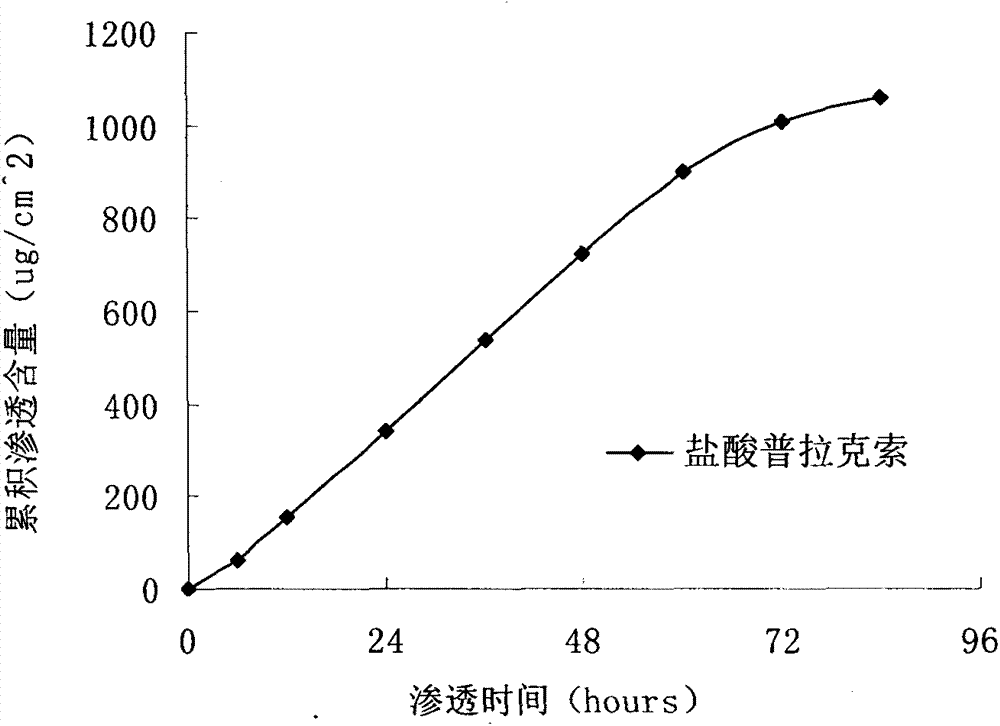
[0030] Preparation method: fully swell polyvinyl alcohol and polyvinyl pyrrolidone with 50% ethanol, add eucalyptus oil, Tween 80, azone, propylene glycol and glycerin, dissolve and mix with m...
Embodiment 2
[0033] The first layer (close to the anti-adhesive layer) composition: polyvinyl alcohol (PVA17-88) 2.0g, polyvinylpyrrolidone (PVP) 0.4g, N-methylpyrrolidone 0.2g, propylene glycol 0.3g, glycerin 0.3g, vitamin C0.2g, pramipexole hydrochloride 15mg.
[0034]The composition of the second layer (middle layer): polyvinyl alcohol (PVA17-88) 2.8g, polyvinylpyrrolidone (PVP) 0.2g, N-methylpyrrolidone 0.4g, propylene glycol 0.3g, glycerin 0.4g, vitamin C0.2g, hydrochloric acid Pramipexole 30mg.
[0035] The composition of the third layer (close to the backing layer): polyvinyl alcohol (PVA17-88) 1.4g, polyvinylpyrrolidone (PVP) 0.3g, N-methylpyrrolidone 0.2g, propylene glycol 0.2g, glycerin 0.2g, vitamin C0. 3g, pramipexole hydrochloride 45mg.
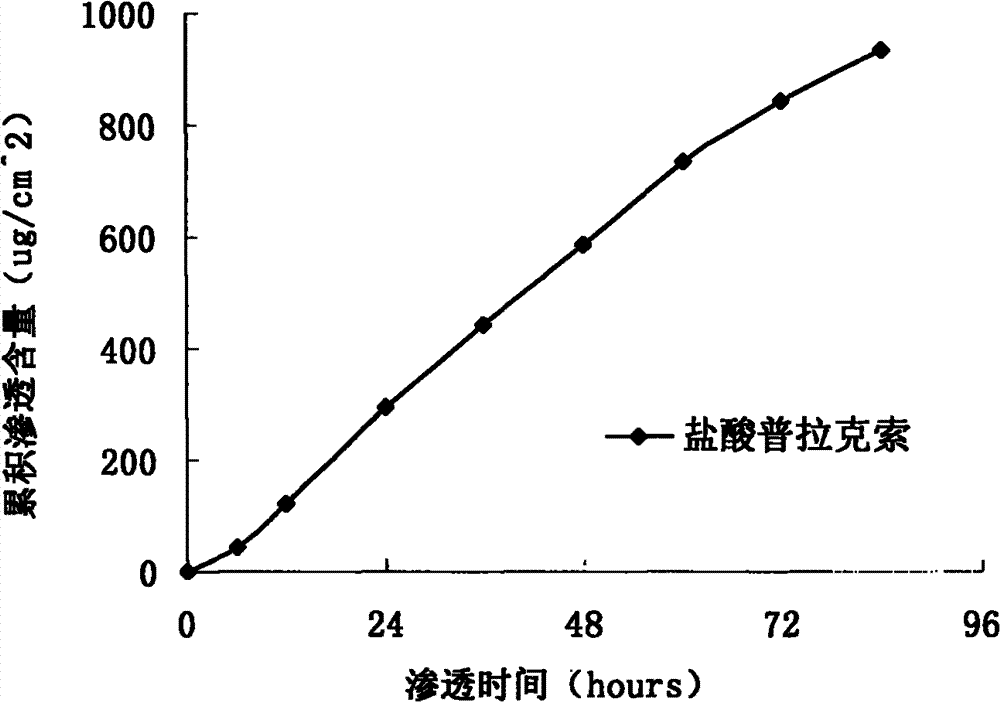
[0036] Prepared as in Example 1, prepared to contain pramipexole hydrochloride 1.84mg / cm 2 transdermal patch. A modified Franz diffusion cell was used to measure the penetration rate of the transdermal patch in the abdominal skin of the h...
Embodiment 3
[0038] The first layer (close to the anti-adhesive layer) composition: polyvinyl alcohol (PVA17-88) 0.6g, polyvinylpyrrolidone (PVP) 0.2g, menthol 0.2g, Tween 800.1g, glycerin 0.4g, sodium metabisulfite 0.05g, pramipexole hydrochloride 1.6mg.
[0039] The composition of the second layer (middle layer): polyvinyl alcohol (PVA17-88) 1.8g, polyvinylpyrrolidone (PVP) 0.3g, menthol 0.25g, Tween 800.1g, glycerin 0.5g, sodium metabisulfite 0.05g, general hydrochloride Laxole 6.4mg.
[0040] The composition of the third layer (close to the backing layer): polyvinyl alcohol (PVA17-88) 0.8g, polyvinylpyrrolidone (PVP) 0.1g, menthol 0.2g, Tween 800.1g, glycerin 0.4g, sodium metabisulfite 0.05g , pramipexole hydrochloride 3.2mg.
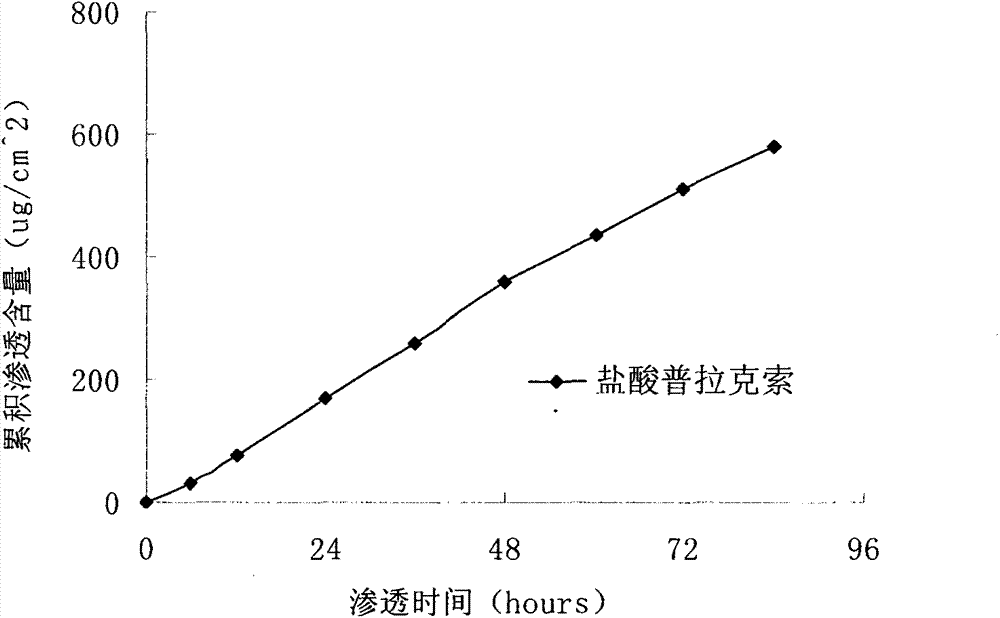
[0041] Prepared as in Example 1, prepared to contain pramipexole hydrochloride 2.04mg / cm 2 transdermal patch. A modified Franz diffusion cell was used to measure the penetration rate of the transdermal patch in the abdominal skin of the hair-free rats, and t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com