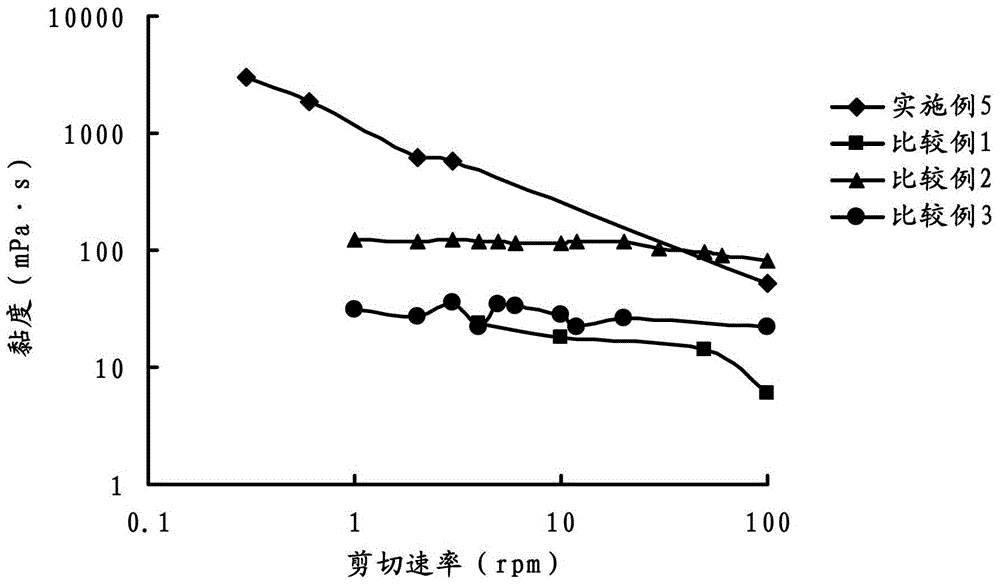
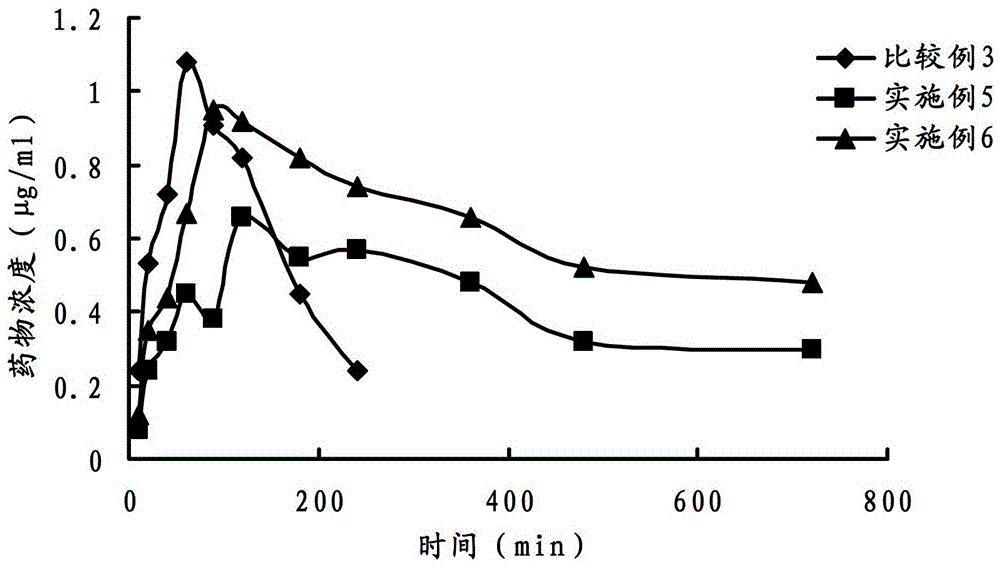
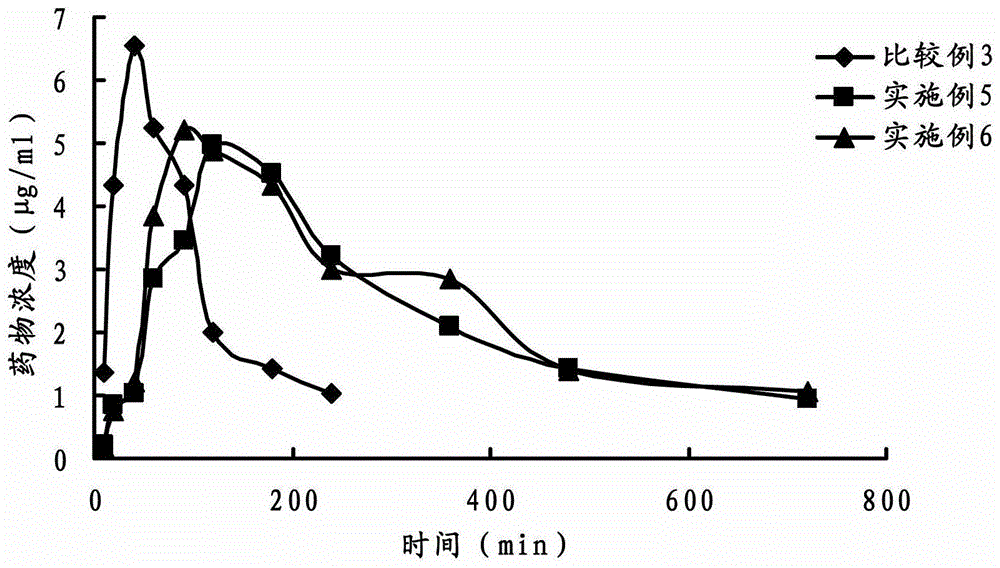
Ophthalmic gel containing dorzolamide and timolol and preparation method thereof
A technology of ophthalmic gel and dorzolamide, applied in the field of pharmaceutical preparations, can solve the problems of reducing the dosage of active ingredients and strong ocular retention
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0096] Embodiment 1: Preparation of ophthalmic gel 1
[0097] Ophthalmic gel 1 was prepared according to the corresponding prescription ratio in Table 1 below.
[0098]Preparation method: (1) Swell the prescribed amount of carbomer, sodium hyaluronate, and polyvinyl alcohol with an appropriate amount of water for injection, and sterilize for later use; (2) Fully mix the carbomer base and polyvinyl alcohol base, and set aside; Dissolve sodium hydroxide in appropriate amount of water, sterilize with 0.22 μm filter and mix with the mixed matrix obtained in (2) for later use; (4) take 40ml of water for injection at 50-80°C, dissolve dorzolamide hydrochloride and timolol maleate in sequence Er, glycerin, ethylparaben, sterilized by 0.22 μm filtration, and set aside; (5) mix the sodium hyaluronate matrix obtained from (1) with the solution obtained from (4) evenly; (6) mix the material obtained from (5) with the material obtained from (3), and add water for injection to 100g, Sti...
Embodiment 2-4
[0099] Embodiment 2-4: Preparation of ophthalmic gel 2-4
[0100] Prepare ophthalmic gels 2-4 according to the corresponding prescription ratio in Table 1.
[0101] The preparation method refers to Example 1.
[0102] Table 1: Prescription ratio
[0103] Component Example 1 Example 2 Example 3 Example 4 Dorzolamide Hydrochloride 0.56g 0.56g 1.11g 2.22g timolol maleate 0.68g 0.34g 0.14g 0.068g Carbomer 980 0.5g 0.5g - - Carbomer 974 - - 0.6g 0.7g sodium hyaluronate 0.7g 0.5g 0.6g 0.6g polyvinyl alcohol 1.1g 0.7g 1.0g 1.2g
[0104] Propylene Glycol - - 1.4g 0.9g glycerin 1.8g 2.0g - - Benzalkonium chloride - 0.01 - 0.005 Ethylparaben 0.03 - 0.03 0.02 sodium hydroxide Appropriate amount Appropriate amount Appropriate amount Appropriate amount Add water for injection to 100g 100g 100g 100g
Embodiment 5-8
[0105] Embodiment 5-8: Preparation of ophthalmic gel 5-8
[0106] Prepare ophthalmic gels 5-8 according to the corresponding prescription ratio in Table 2.
[0107] Table 2: Prescription ratio
[0108]
[0109] Preparation method: (1) Swell the prescribed amount of carbomer, sodium hyaluronate, and polyvinyl alcohol with an appropriate amount of water for injection, and sterilize for later use; (2) Fully mix the carbomer base and polyvinyl alcohol base, and set aside; Dissolve sodium hydroxide in appropriate amount of water, sterilize with 0.22 μm filter and mix with the mixed matrix obtained in (2) for later use; (4) take 40ml of water for injection at 50-80°C, dissolve dorzolamide hydrochloride and timolol maleate in sequence Alcohol, glycerin, ethylparaben, HS15, sterilized by 0.22μm filtration, and set aside; (5) mix the sodium hyaluronate matrix obtained from (1) with the solution obtained from (4) evenly; (6) mix the material obtained from (5) with the material o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
| alcoholysis degree | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com