Preparation method of high-purity pramipexole dihydrochloride
A high-purity technology for pramipexole hydrochloride, which is applied in the field of preparation of high-purity pramipexole hydrochloride, can solve problems such as low reaction yield, cumbersome process, and loss of crystal water, and achieve simple reaction steps, high safety, water a stable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
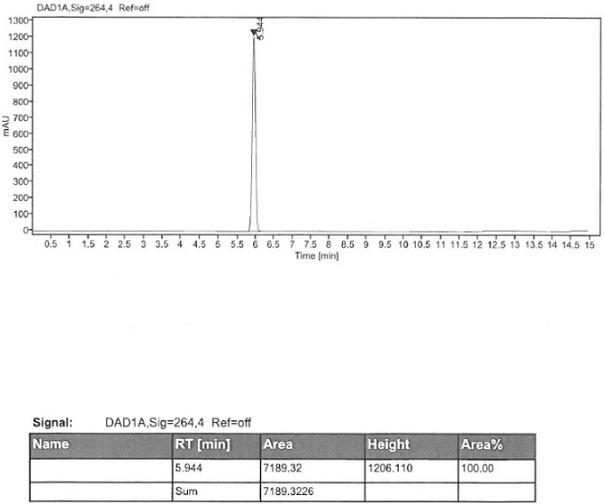
[0042] Add 50 g of (S)-2-amino-6-propionamido-4,5,6,7-tetrahydrobenzothiazole, 300 ml of tetrahydrofuran, and 20 g of sodium borohydride into the reaction flask. 94.5 g of boron trifluoride was added dropwise at a temperature of 10 degrees Celsius, and after the drop was completed, the temperature was raised to reflux for 2 hours of reaction. After cooling down to room temperature, dilute hydrochloric acid was added to terminate the reaction. Add 20% sodium hydroxide aqueous solution, adjust the pH to 14, add ethyl acetate for extraction, and separate layers. The organic layer was concentrated until 4-5V of solvent remained, then 2V of methanol was added, 0.75V of concentrated hydrochloric acid was added dropwise to form a salt, and the temperature was lowered to 10°C for crystallization. After filtration and drying, 60.3 g of pramipexole hydrochloride dihydrochloride monohydrate was obtained, with a molar yield of 89.9% and a purity of 99.92%.
[0043] Add 50g of pramipexol...
Embodiment 2
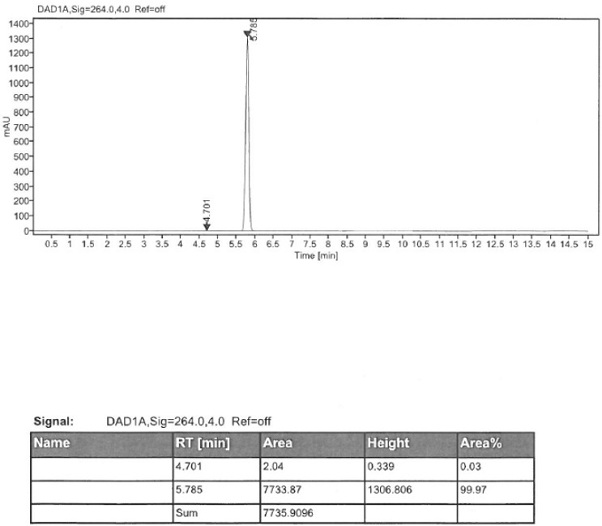
[0045] Add 50 g of (S)-2-amino-6-propionamido-4,5,6,7-tetrahydrobenzothiazole, 400 ml of tetrahydrofuran, and 24 g of sodium borohydride into the reaction flask. 113.4 g of boron trifluoride was added dropwise at a temperature of 10 degrees Celsius, and after the drop was completed, the temperature was raised to reflux for 2 hours of reaction. After cooling down to room temperature, dilute hydrochloric acid was added to terminate the reaction. Add 20% sodium hydroxide aqueous solution, adjust the pH to 14, add ethyl acetate for extraction, and separate layers. The organic layer was concentrated until 4-5V of solvent remained, and then 2V of methanol was added, and 0.8V of concentrated hydrochloric acid was added dropwise to form a salt, and the temperature was lowered to 10°C for crystallization. After filtration and drying, 61.5 g of pramipexole hydrochloride dihydrochloride monohydrate was obtained, with a molar yield of 91.7% and a purity of 99.91%.
[0046] Add 50g of pr...
Embodiment 3
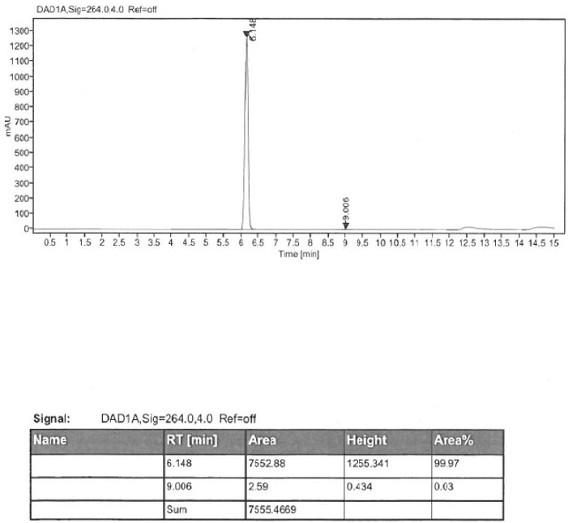
[0048] Add 50 g of (S)-2-amino-6-propionamido-4,5,6,7-tetrahydrobenzothiazole, 500 ml of tetrahydrofuran, and 22 g of sodium borohydride into the reaction flask. 104 g of boron trifluoride was added dropwise at a temperature of 10 degrees Celsius, and after the drop was completed, the temperature was raised to reflux for 2 hours of reaction. After cooling down to room temperature, dilute hydrochloric acid was added to terminate the reaction. Add 20% sodium hydroxide aqueous solution, adjust the pH to 14, add ethyl acetate for extraction, and separate layers. The organic layer was concentrated until 4-5V of solvent remained, and then 2V of methanol was added, and 0.8V of concentrated hydrochloric acid was added dropwise to form a salt, and the temperature was lowered to 10°C for crystallization. After filtration and drying, 61.1 g of pramipexole hydrochloride dihydrochloride monohydrate was obtained, with a molar yield of 91.1% and a purity of 99.91%.
[0049] Add 50g of pram...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com