Pramipexole dihydrochloride solution prepared from pramipexole dihydrochloride solid preparation and determination method thereof
A technology of pramipexole hydrochloride and solid preparations, which is applied in the field of pramipexole hydrochloride solution and its determination, which can solve the problems of unavailable, expensive enzyme solution, and difficult disintegration of tablets, and achieve high accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
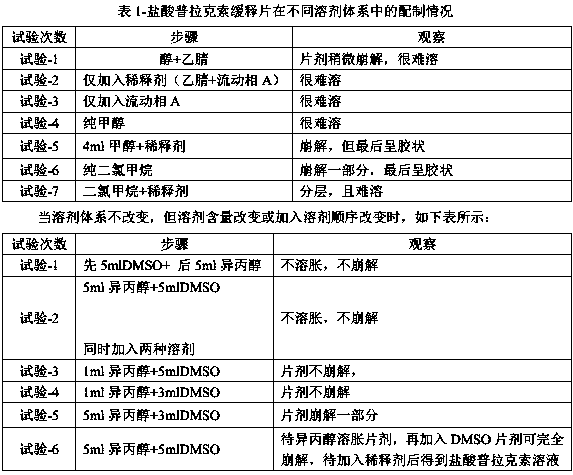
[0026] Preparation of pramipexole hydrochloride solution of the present invention (sample specification: API: 0.26 mg): Take 5 pramipexole sustained-release tablets, weigh them precisely, place them in a 500ml measuring bottle, add 25ml of isopropanol, and let stand for 5 minutes. Add 25ml of dimethyl sulfoxide, stir magnetically for 20min, and rotate at 1000rpm; add 350ml of diluent, sonicate for 60min, shake occasionally during sonication, take out the stirring bar, and dilute to the mark with diluent; centrifuge at 9000rpm for 20min. After filtering with a 0.45 μm filter membrane, 3 ml of the initial filtrate was discarded, and the filtrate obtained pramipexole hydrochloride solution.
Embodiment 2
[0032] 1. Experimental materials and instrument conditions
[0033] Experimental materials: acetonitrile, manufacturer: Rankem; phosphoric acid, manufacturer: Merck; potassium dihydrogen phosphate, manufacturer: Merck; anhydrous 1-octanesulfonic acid sodium salt, manufacturer: Rankem; dimethyl sulfoxide, manufacturer Manufacturer: Rankem; isopropanol, manufacturer: Merck; ultrapure water, manufacturer: Zhuhai Rundu Pharmaceutical Co., Ltd.; pramipexole hydrochloride sustained-release tablets, manufacturer: Zhuhai Rundu Pharmaceutical Co., Ltd., specification: 0.26 mg, 0.58mg, 1.05mg.
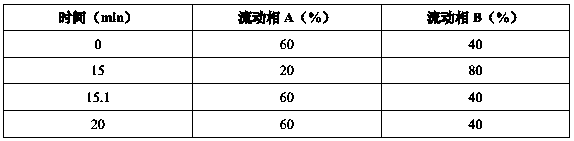
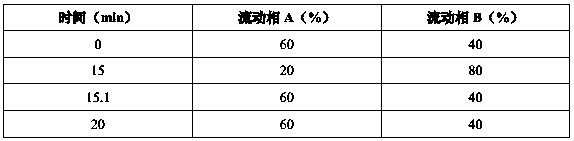
[0034] Instrument: high performance liquid chromatography: Agilent 1260; ultrasonic instrument: pH meter: Lab India-PICO+; semi-micro balance: Radwag-XA 82 / 220 / 2X; ultrasonic instrument: PCI analytics; constant temperature oscillator: Glass co; centrifuge : Eltek; electronic analytical balance: XSE205DU, GR-200; chromatographic column: Inertsil ODS-3V (150×4.6mm), 5 μm.
[0035] Detection meth...
Embodiment 3
[0048] Embodiment 3 Detection method specificity test of the present invention
[0049] Specificity is to examine the identification and selectivity of the components to be tested. It is required that the blank solution and blank excipients do not interfere with the detection of pramipexole hydrochloride; in the system adaptability solution, all impurity peaks are well separated from the chromatographic peaks of pramipexole hydrochloride .
[0050] Interference assessment of blank solution and blank excipient solution: Analyze the blank solution (diluent), reference solution and blank excipient solution according to the assay method.
[0051] Conclusion: The number of theoretical plates is not less than 2000, the tailing factor is not more than 2.0, the blank solution has no interference at the retention time of pramipexole, the blank excipient solution has no interference at the retention time of pramipexole hydrochloride, and all impurity peaks in the sample solution are It...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com