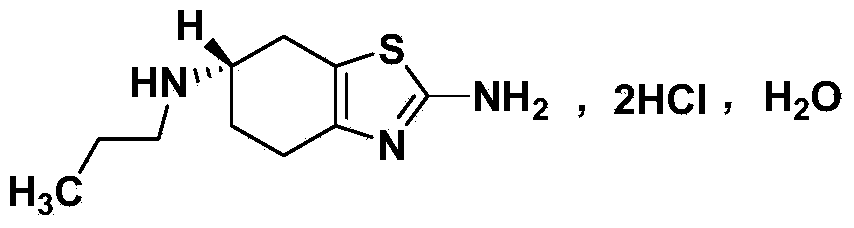
Method for detecting enantiomer in pramipexole dihydrochloride and method for separating enantiomer from pramipexole dihydrochloride
A technology of pramipexole hydrochloride and enantiomers, which is applied in the detection of enantiomers in pramipexole hydrochloride and the separation of the two, can solve the problems of poor resolution and unsatisfactory separation effect, etc. To achieve the effect of ensuring quality control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
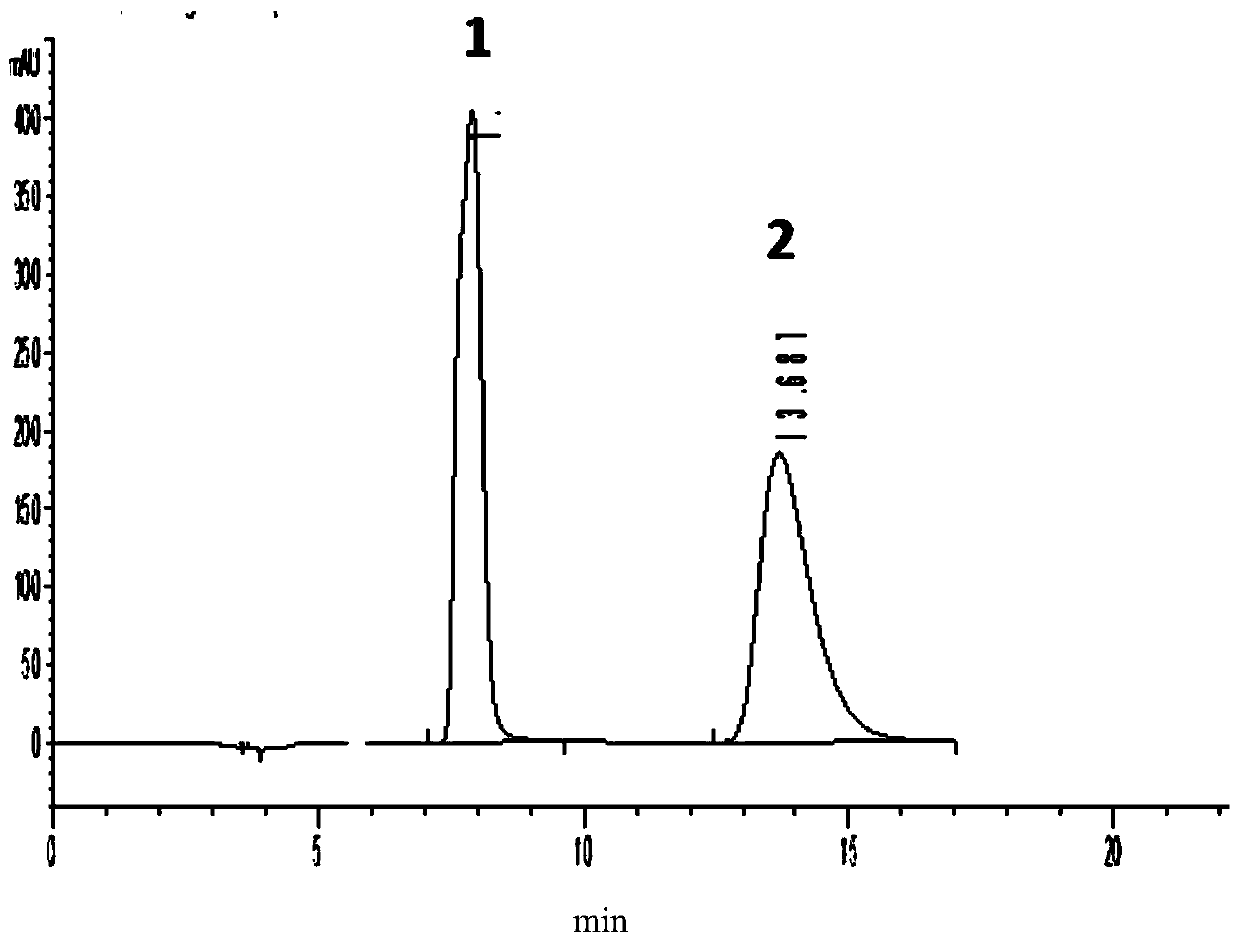
[0031] Example 1 Analysis and detection of pramipexole hydrochloride and its enantiomers
[0032] Instrument and chromatographic conditions:
[0033] High performance liquid chromatograph Agilent 1260 (including VWD detector, Agilent chromatographic workstation);
[0034] Chromatographic column: Chiralpak AY-H (Daicel, 250mm×4.6mm, 5μm);
[0035] Mobile phase: n-hexane - isopropanol - diethylamine (85:15:0.1);
[0036] Flow rate: 1.0ml / min;
[0037] Detection wavelength: 254nm;
[0038] Column temperature: 35°C;
[0039] Injection volume: 75 μL.
[0040] Experimental steps:
[0041] Take 6 mg of pramipexole hydrochloride racemate (containing pramipexole hydrochloride and its enantiomers) sample, put it in a 20mL volumetric flask, add 5mL of absolute ethanol to dissolve the sample by ultrasonic, use the mobile phase to make the volume up to the mark, Become a racemate sample solution. Take another 6 mg of pramipexole hydrochloride (S-configuration) sample, put it in a 2...
Embodiment 2
[0045] Embodiment 2 The influence of mobile phase ratio on separation effect
[0046] Instrument and chromatographic conditions:
[0047] High performance liquid chromatograph Agilent 1260 (including VWD detector, Agilent chromatographic workstation);
[0048] Chromatographic column: Chiralpak AY-H (Daicel, 250mm×4.6mm, 5μm);
[0049] Flow rate: 1.0ml / min;
[0050] Detection wavelength: 254nm;
[0051] Column temperature: 35°C;
[0052] Injection volume: 75 μL.
[0053] Experimental steps:
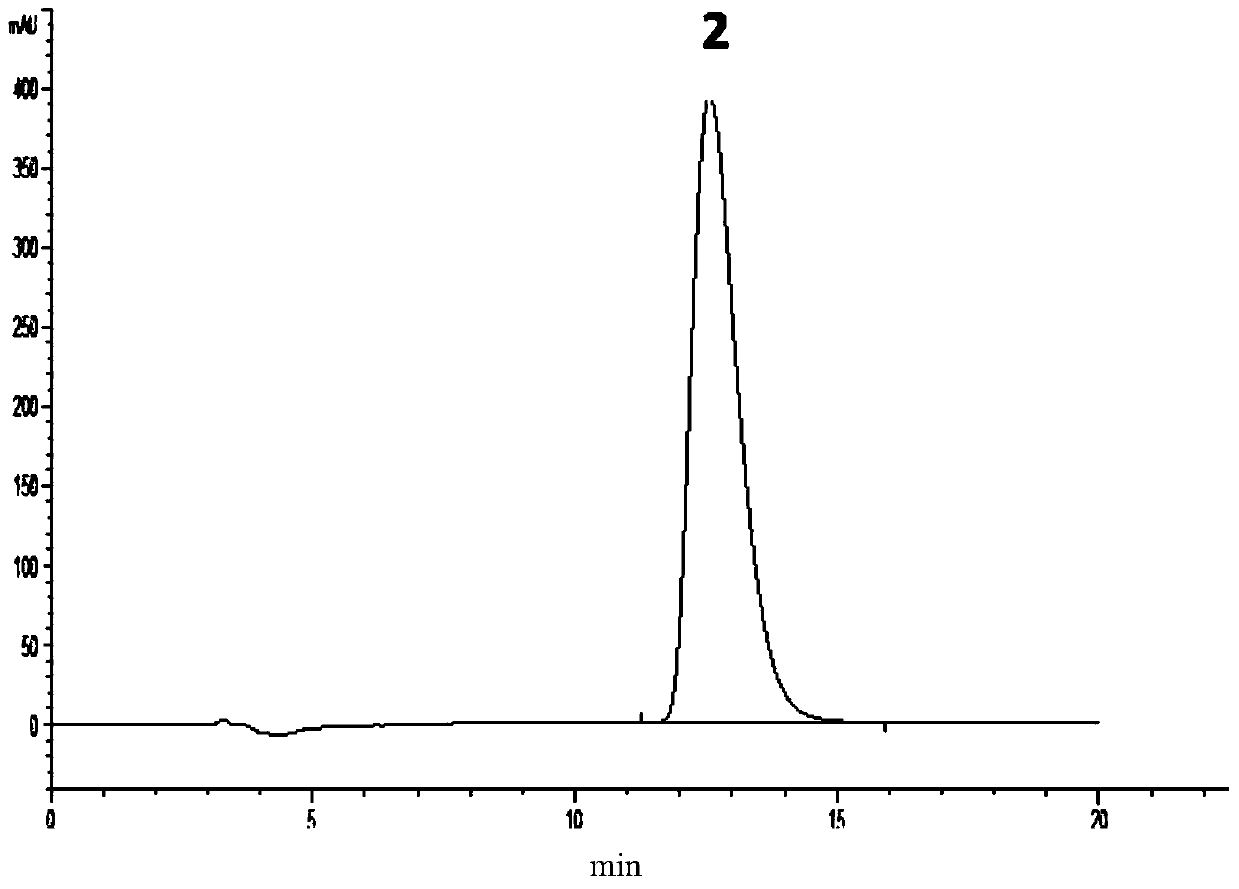
[0054] Take 6mg of pramipexole hydrochloride racemate sample, place it in a 20mL volumetric flask, add 5mL of absolute ethanol to dissolve the sample ultrasonically, and dilute to the mark with mobile phase to become a racemate sample solution. According to the above chromatographic conditions, adjust the ratio of n-hexane containing 0.1% diethylamine to isopropanol to be 90:10, 85:15, and 80:20 respectively, carry out HPLC analysis on the racemate sample solution, and investigate th...
Embodiment 3
[0059] The influence of embodiment 3 flow velocity on separation effect
[0060] Instrument and chromatographic conditions:
[0061] High performance liquid chromatograph Agilent 1260 (including VWD detector, Agilent chromatographic workstation);
[0062] Chromatographic column: Chiralpak AY-H (Daicel, 250mm×4.6mm, 5μm);
[0063] Mobile phase: n-hexane - isopropanol - diethylamine (85:15:0.1);
[0064] Detection wavelength: 254nm;
[0065] Column temperature: 35°C;
[0066] Injection volume: 75 μL.
[0067] Experimental steps:
[0068] Take 6 mg of pramipexole hydrochloride racemate sample, place it in a 20mL volumetric flask, add 5mL of absolute ethanol to dissolve the sample ultrasonically, and dilute to the mark with mobile phase to become a racemate sample solution. According to the above-mentioned chromatographic conditions, the flow rate is adjusted to be 0.8ml / min, 1.0ml / min and 1.2ml / min, and the racemate sample solution is analyzed by high performance liquid chrom...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com