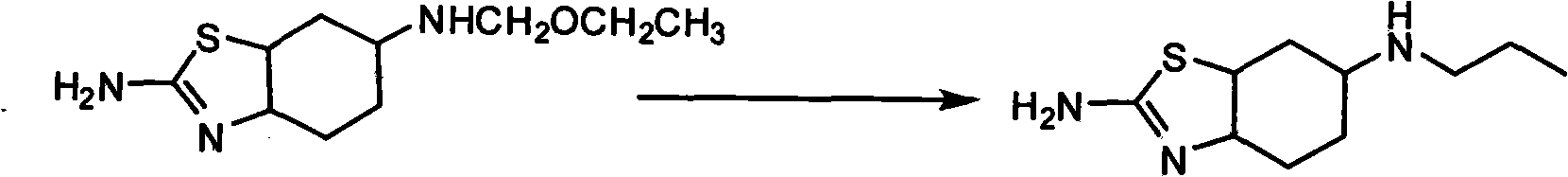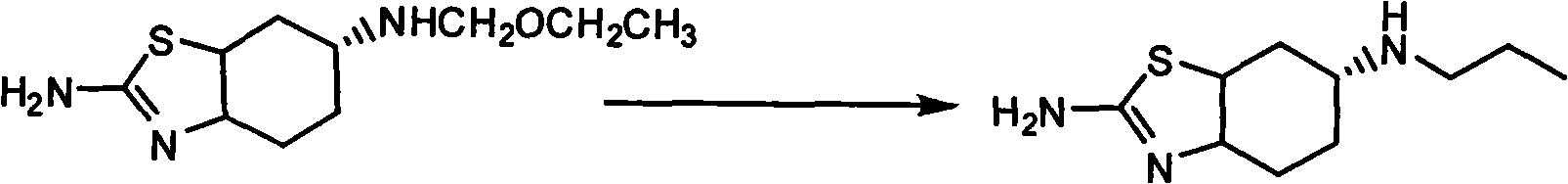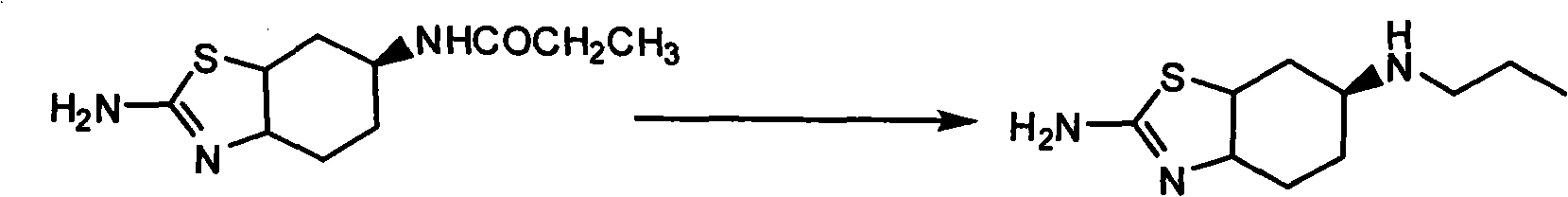Preparation method for preparing intermediate body of Pramipexole dihydrochloride
A technology for pramipexole hydrochloride and intermediates, which is applied in the field of preparation of pramipexole hydrochloride, and can solve the problems of low yield, unsuitability for industrial production, and high reagent prices
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 Preparation of 2-amino-6-propylamino-4,5,6,7-tetrahydrobenzothiazole
[0032] In the reaction flask, add THF150ml, ZnCl 2 (17.95g, 0.132mol) and KBH 4 (14.25g, 0.264mol), under nitrogen protection, stirred at room temperature for 2 hours;
[0033] Add 2-amino-6-propionamido-4,5,6,7-tetrahydrobenzothiazole (15g, 0.066mol) and 70ml of toluene, heat slowly, evaporate part of the solvent, make the internal temperature reach 98°C, keep warm Stir for 4 hours. Cool to room temperature, pour into 150ml weight concentration of 10% HCl, the pH value of the reaction system is about 2, filter, the filtrate is extracted with chloroform, and alkalized to pH 12 with 20% weight concentration of sodium hydroxide solution. Extract with chloroform 75ml×3, combine the extracts with NaSO 4 Dry and recover chloroform to obtain 13.2 g of 2-amino-6-propylamino-4,5,6,7-tetrahydrobenzothiazole, yield 95%, m.p.>280°C, MS (m / z): 211.11, HPLC: 98.5%.
Embodiment 2
[0034] Example 2 Preparation of S-(-)-2-amino-6-propylamino-4,5,6,7-tetrahydrobenzothiazole
[0035] In the reaction flask, add 150ml of 2-methyltetrahydrofuran, ZnCl 2 (8.97g, 0.066mol) and KBH 4 (7.13g, 0.132mol), under nitrogen protection, stirred at room temperature for 4 hours. Add S-(-)-2-amino-6-propionamido-4,5,6,7-tetrahydrobenzothiazole (15g, 0.066mol) and 70ml of toluene, heat slowly, evaporate part of the solvent, and make the inner temperature reached 93°C, and kept stirring for 6 hours. Cool to room temperature, pour into 150ml of sulfuric acid with a weight concentration of 10%, the pH value of the reaction solution is between about 1, filter, and after the filtrate is extracted with dichloromethane, it is alkalized with a 20% potassium hydroxide solution by weight to The pH is 13. Extract with dichloromethane 80ml×3, combine the extracts with NaSO 4 Dry and recover dichloromethane to obtain 10.4 g of S-(-)-2-amino-6-propylamino-4,5,6,7-tetrahydrobenzothiaz...
Embodiment 3
[0036] Example 3 Preparation of R-(+)-2-amino-6-propylamino-4,5,6,7-tetrahydrobenzothiazole
[0037] In the reaction flask, add THF200ml, ZnCl 2 (6.8g, 0.05mol) and KBH 4(15.4 g, 0.1 mol), under nitrogen protection, stirred at room temperature for 2 hours. Add R-(+)-2-amino-6-propionamido-4,5,6,7-tetrahydrobenzothiazole (7.5g, 0.033mol) and 100ml of benzene, heat slowly, evaporate part of the solvent, and make When the internal temperature reached 85° C., the mixture was kept stirring for 4 hours. Cool to room temperature, pour into 200ml of 10% HCl by weight, the pH value of the reaction solution is 1, filter, and after the filtrate is extracted with chloroform, basify with 20% sodium hydroxide solution by weight until the pH is 12. Extract with chloroform 50ml×3, combine the extracts with NaSO 4 Dry and recover chloroform to obtain 8.5 g of R-(+)-2-amino-6-propylamino-4,5,6,7-tetrahydrobenzothiazole, yield 61%, m.p.>280°C, MS (m / z): 211.11, HPLC: 98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com