Compound and preparation method thereof
A technology of compounds and hydrates, applied in the field of compounds and their preparation, can solve the problems of complex reactions, harsh reaction conditions, and low purity, and achieve the effect of simple reaction process, mild reaction conditions, and easy separation and purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
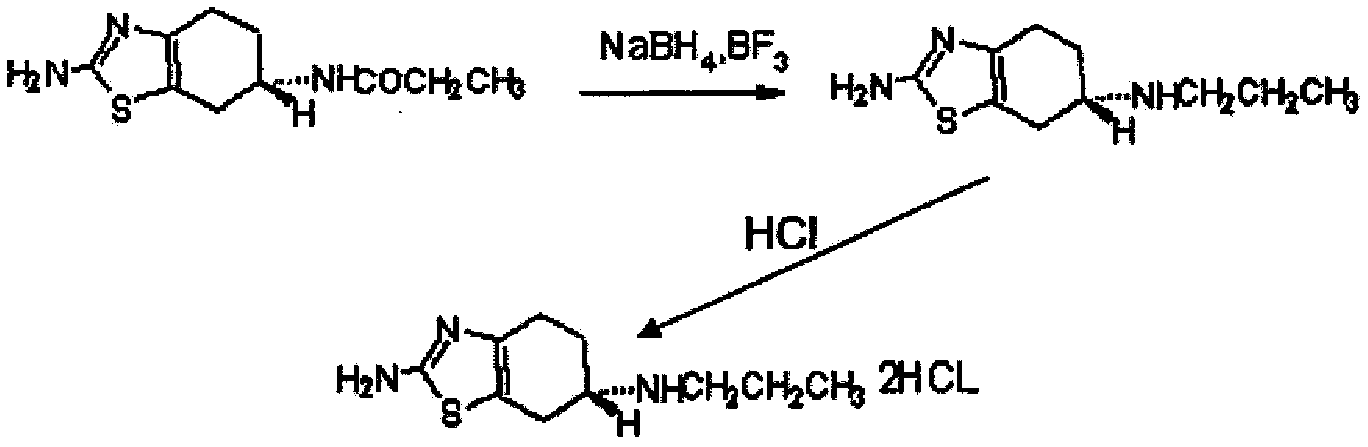
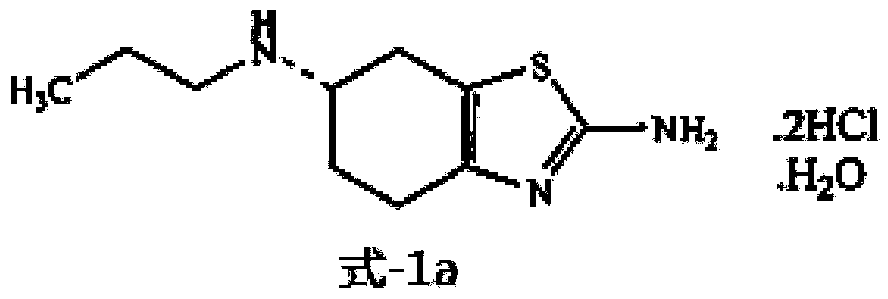
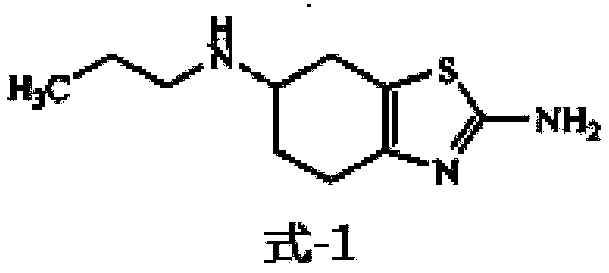
[0061]Put 40.00g (0.236mol, 1.0 molar equivalent) of the starting material into a 1000ml three-necked reaction flask, add 160.0ml tetrahydrofuran, then add 19.85g (0.236mol, 1.0 molar equivalent) sodium bicarbonate, stir and heat up to 50°C, Add 29.22g (0.225mol, 0.95 mole equivalent) of propionic anhydride dropwise, control the dropwise addition within 1 hour, stir and react for 0.5 hour after the dropwise addition, then cool down to 5±5°C, then add 26.82g (0.708mol, 3.0mol Equivalent) sodium borohydride, dropwise add boron trifluoride ether solution 118.4ml (0.944mol, 4.0 molar equivalent, containing 47.0% boron trifluoride, density 1.15g / ml, Zibo Linzi Xinqiang Chemical Co., Ltd., batch number: 20121008 ), control the dropwise addition in 1 hour, keep stirring for 1 hour after the dropwise addition, naturally rise to room temperature and stir for 1.5 hours, reflux and stir for 1.5 hours; cool down to 10±5°C, add 120.0ml of water dropwise, and add 100.0ml of water after 20 mi...
Embodiment 2
[0064] Put 40.00g (0.236mol, 1.0 molar equivalent) of the starting material into a 2000ml three-necked reaction flask, add 320.0ml of tetrahydrofuran, then add 47.26g (0.472mol, 2.0 molar equivalent) of potassium bicarbonate, stir and heat up to reflux, Start to add 30.76g (0.236mol, 1.0 molar equivalent) of propionic anhydride dropwise, control the dropwise addition within 2 hours, stir and react for 1 hour after the dropwise addition, then cool down to 5±5°C, then add 53.64g (1.416mol, 6.0 Molar equivalent) sodium borohydride, dropwise added boron trifluoride ether solution 207.2ml (1.652mol, 7.0 molar equivalent, containing 47.0% boron trifluoride, density 1.15g / ml, Zibo Linzi Xinqiang Chemical Co., Ltd., batch number: 20121008), control the dropwise addition in 5 hours, keep stirring for 2 hours after the dropwise addition, naturally rise to room temperature and stir for 3 hours, reflux and stir for 3 hours; cool down to 10±5°C, add 120.0ml of water dropwise, and add after ...
Embodiment 3
[0067] Put 40.00g (0.236mol, 1.0 molar equivalent) of the starting material into a 1000ml three-necked reaction flask, add 200ml of tetrahydrofuran, then add 23.82g (0.284mol, 1.2 molar equivalent) of sodium bicarbonate, stir and heat up to reflux, and start Add 30.76g (0.236mol, 1.0 molar equivalent) propionic anhydride dropwise (propionic anhydride is added to 120ml tetrahydrofuran to dilute), control the dropwise addition in 1 hour, stir the reaction for 0.5 hour after the dropwise addition, cool down to 5±5°C, and then add 30.8g (0.813mol, 3.4 molar equivalent) sodium borohydride, dropwise added boron trifluoride ether solution 136.2ml (1.086mol, 4.6 molar equivalent, containing 47.0% boron trifluoride, density 1.15g / ml, Linzixin, Zibo City Strong Chemical Co., Ltd., batch number: 20121008), control the dropwise addition within 3 hours, keep stirring for 1 hour after the dropwise addition, naturally rise to room temperature and stir for 2 hours, reflux and stir for 2 hours;...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com