Pramipexole dihydrochloride sustained release preparation and preparation method thereof
A technology of pramipexole hydrochloride and sustained-release preparations, which is applied in the direction of pharmaceutical formulas, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., which can solve the problem of low uniformity of sustained-release preparations and achieve improved release The effect of degree and uniformity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
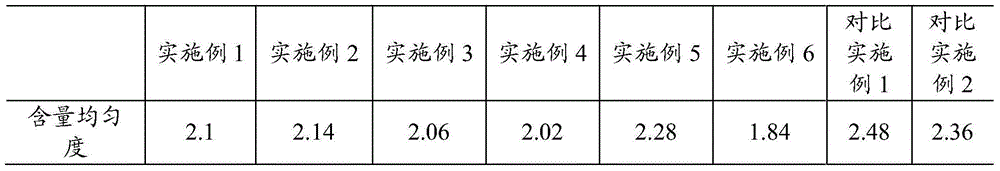
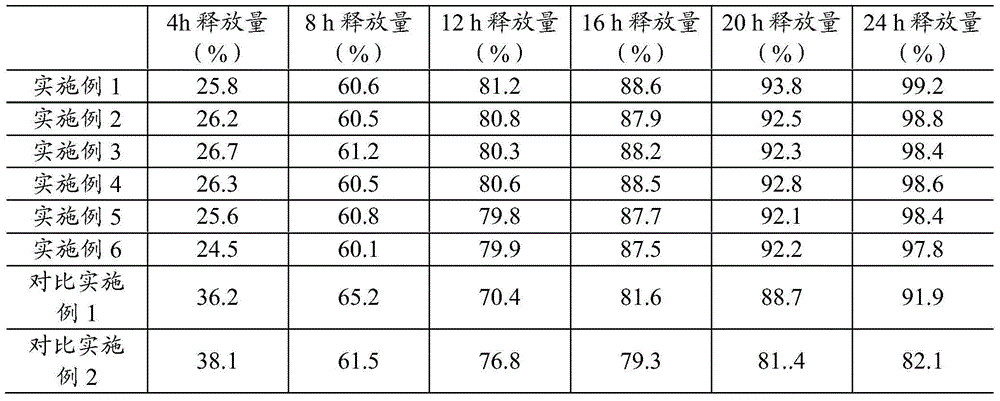
Embodiment 1
[0019] A kind of pramipexole hydrochloride sustained-release preparation, is made up of mixed matrix tablet and the coating layer that wraps described mixed matrix tablet, and described mixed matrix tablet is made up of following components (parts by weight): 5 parts of pramipexole hydrochloride, hydroxyl 30 parts of propylmethylcellulose, 20 parts of wax, and the coating layer includes the following components (parts by weight): 50 parts of compressible starch, 50 parts of microcrystalline cellulose, and 50 parts of talcum powder. Its preparation method is as follows:
[0020] 1) Mix pramipexole hydrochloride and hydroxypropyl methylcellulose evenly, add 200 parts of purified water, place in a centrifugal pellet machine, and use 100 parts of purified water to centrifugally make pellets by spraying liquid and adding powder, and then use wax 20 parts by weight are skeleton materials to make the mixed skeleton tablet.
[0021] 2) Mix and stir the compressible starch, the talc p...
Embodiment 2
[0024] A kind of pramipexole hydrochloride sustained-release preparation, is made up of mixed matrix tablet and the coating layer that wraps described mixed matrix tablet, and described mixed matrix tablet is made up of following components (parts by weight): 10 parts of pramipexole hydrochloride, hydroxyl 80 parts of propylmethylcellulose, 40 parts of wax, and the coating layer includes the following components (parts by weight): 100 parts of compressible starch, 100 parts of microcrystalline cellulose, and 100 parts of micronized silica gel. Its preparation method is as follows:
[0025] 1) Mix pramipexole hydrochloride and hypromellose evenly, add 200 parts of purified water and 200 parts of ethanol, place in a centrifugal pellet machine, use 150 parts of purified water and 150 parts of ethanol to spray liquid and add powder After centrifugal pelletizing, wax is used as the skeleton material to make the mixed skeleton tablet.
[0026] 2) Mix and stir the compressible starc...
Embodiment 3
[0029] A kind of pramipexole hydrochloride sustained-release preparation, is made up of mixed matrix tablet and the coating layer that wraps described mixed matrix tablet, and described mixed matrix tablet is made up of following components (parts by weight): 25 parts of pramipexole hydrochloride, hydroxyl 150 parts of propylmethylcellulose, 80 parts of wax, and the coating layer includes the following components (parts by weight): 200 parts of compressible starch, 200 parts of microcrystalline cellulose, and 150 parts of talcum powder. Its preparation method is as follows:
[0030] 1) Mix pramipexole hydrochloride and hypromellose, extrude strips through a sieve plate with a pore size of 0.5 mm, and use wax as the skeleton material to make the mixed skeleton tablet.
[0031] 2) Mix and stir the compressible starch, the lubricant, and the microcrystalline cellulose with 400 parts of ethanol to uniformly prepare a suspension.
[0032] 3) Put the mixed matrix tablet into a flui...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com