Pramipexole dihydrochloride sustained-release preparation and preparing method thereof
A technology for pramipexole hydrochloride and sustained-release preparations, which is applied in the field of pramipexole hydrochloride sustained-release preparations and its preparation, which can solve the problems of insufficiently stable release and too fast release, achieve uniform and stable content, and avoid different release amounts.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
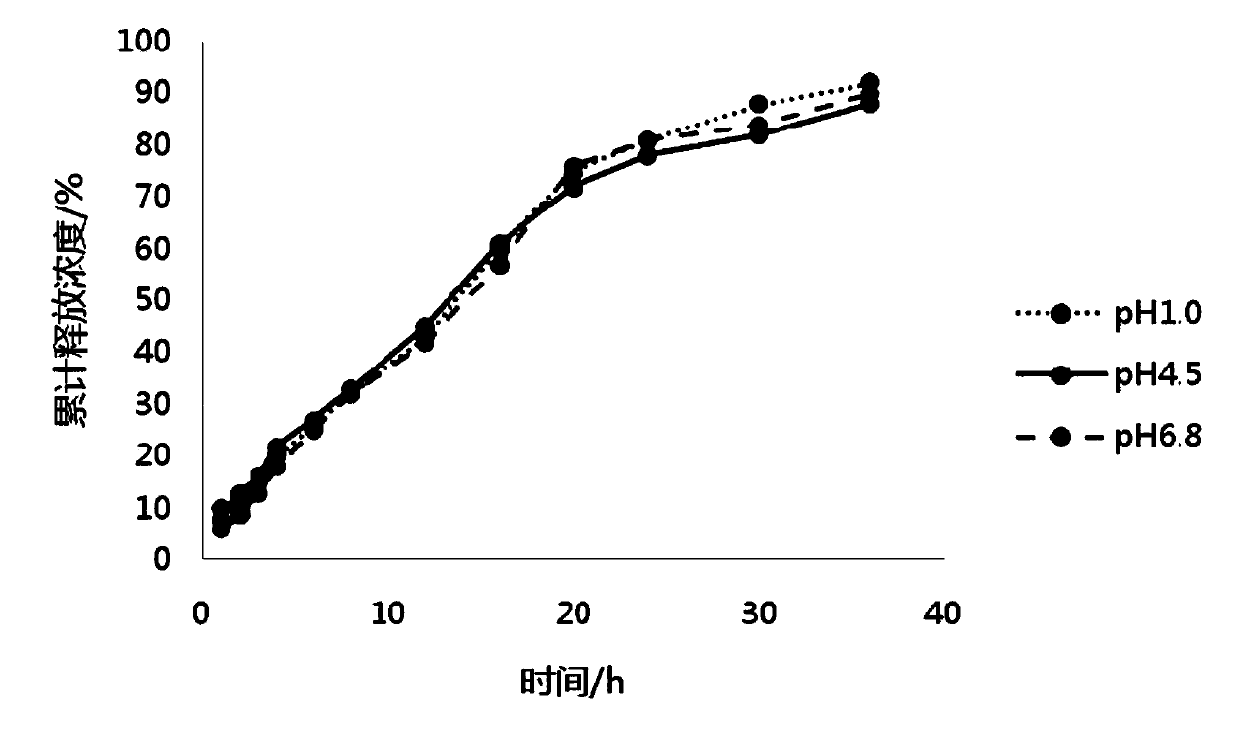
[0043] Embodiment 1 The preparation of pramipexole hydrochloride sustained-release preparation of the present invention
[0044] 1. The dosage of each component of pramipexole hydrochloride sustained-release preparation
[0045] The components and dosages of pramipexole hydrochloride sustained-release preparations in Example 1 are shown in Table 1.
[0046] Components of pramipexole hydrochloride sustained-release preparation in table 1 embodiment 1
[0047] Name of raw material
Dosage per tablet / mg
Weight percentage / %
Dosage / g
pramipexole hydrochloride
0.375
0.15%
0.5625
Hypromellose K15
60
24.00%
90
Eudragit RS
20
8.00%
30
122.125
48.85%
183.1875
Microcrystalline Cellulose KG802
40
16.00%
60
2.5
1.00%
3.75
silica
5
2.00%
7.5
total
250
100.00%
375
[0048] 2. Preparation of Pramipexo...
Embodiment 2
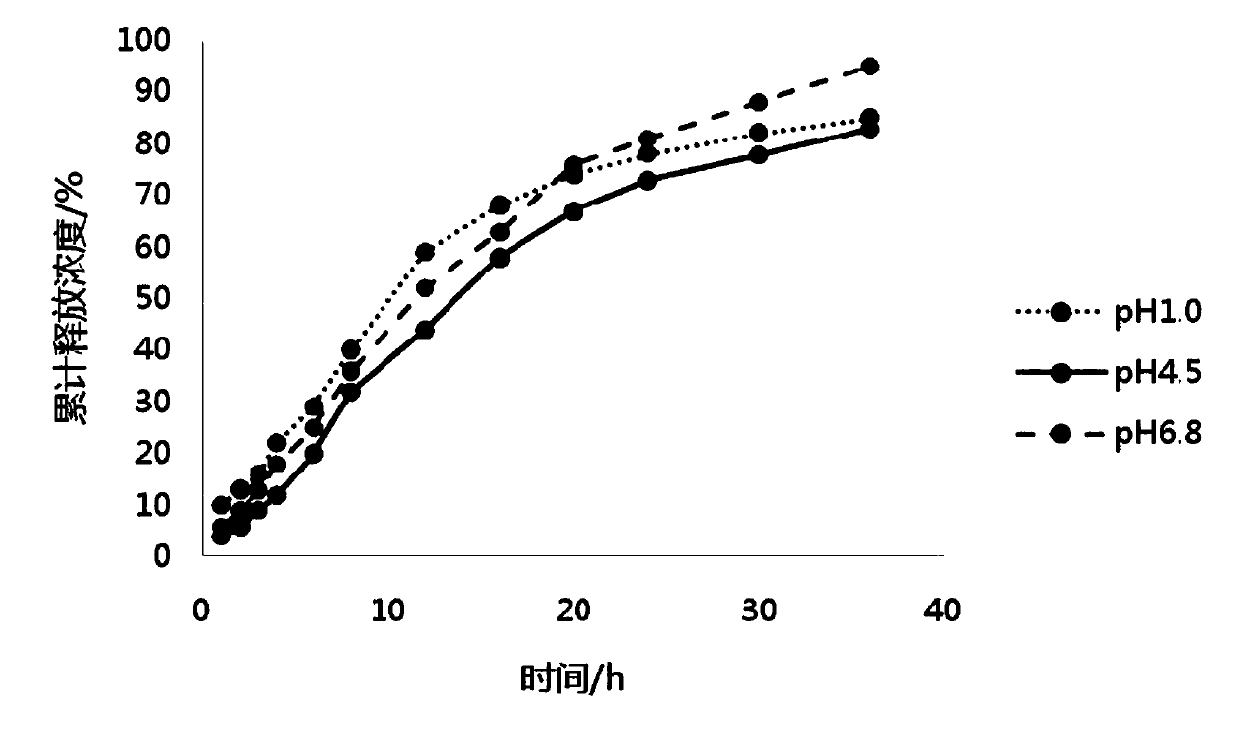
[0055] Embodiment 2 Preparation of pramipexole hydrochloride sustained-release preparation of the present invention
[0056] 1. The dosage of each component of pramipexole hydrochloride sustained-release preparation
[0057] The components and dosages of pramipexole hydrochloride sustained-release preparations in Example 2 are shown in Table 2.
[0058] Components of pramipexole hydrochloride sustained-release preparation in table 2 embodiment 2
[0059] Name of raw material
Dosage per tablet / mg
Percent content / %
Dosage / g
pramipexole hydrochloride
0.375
0.15%
0.5625
40
26.00%
97.5
Carbomer 934P
35
14.00%
52.5
Ethylcellulose-N
25
10.00%
37.5
77.125
30.85%
115.6875
Microcrystalline Cellulose KG802
40
16.00%
60
2.5
1.00%
3.75
silica
5
2.00%
7.5
total
250
...
Embodiment 3
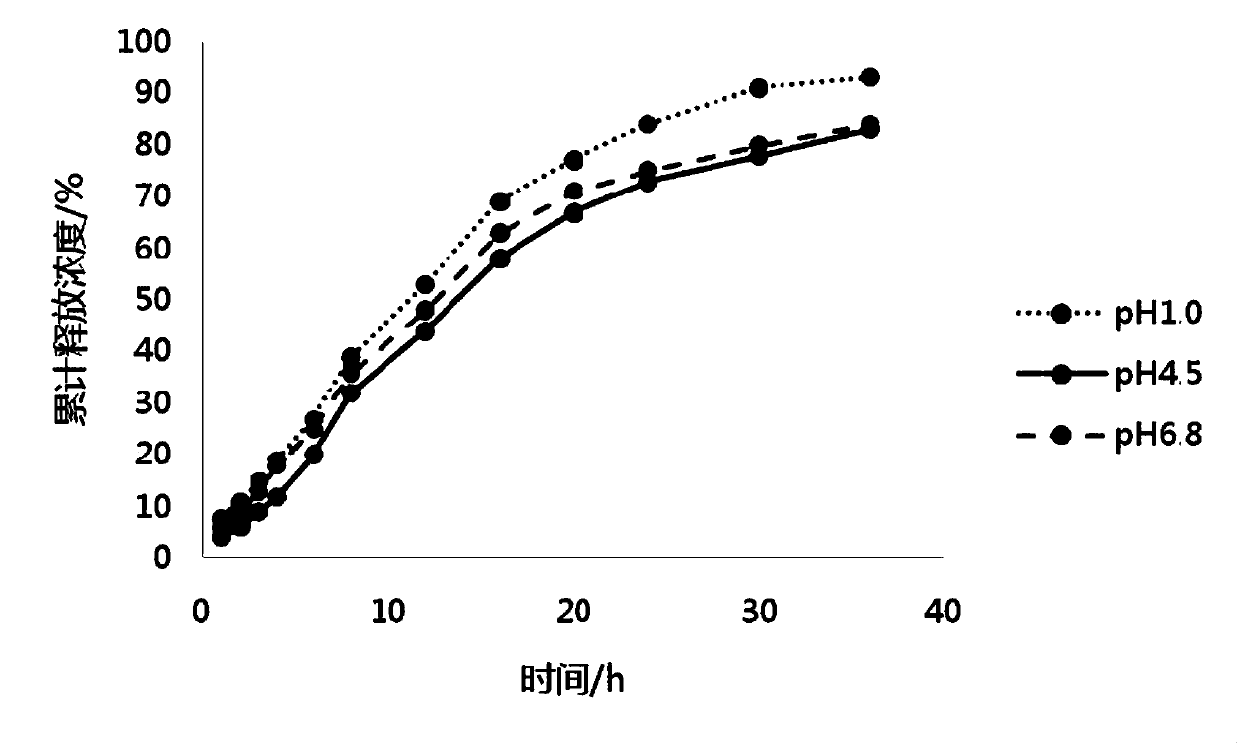
[0067] Embodiment 3 Preparation of pramipexole hydrochloride sustained-release preparation of the present invention
[0068] 1. The dosage of each component of pramipexole hydrochloride sustained-release preparation
[0069] The components and dosages of pramipexole hydrochloride sustained-release preparations in Example 3 are shown in Table 3.
[0070] Components of pramipexole hydrochloride sustained-release preparation in table 3 embodiment 3
[0071] Name of raw material
Dosage per tablet / mg
Percent content / %
Dosage / g
pramipexole hydrochloride
0.375
0.15%
0.5625
Hypromellose K100
25
10.00%
37.5
Eudragit RL100
20
8.00%
30
Ethylcellulose-N
77.125
30.85%
115.6875
Microcrystalline Cellulose PH302
120
48.00%
180
2.5
1.00%
3.75
silica
5
2.00%
7.5
total
250
100.00%
375
[0072] 2. Preparation of Pramipe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com