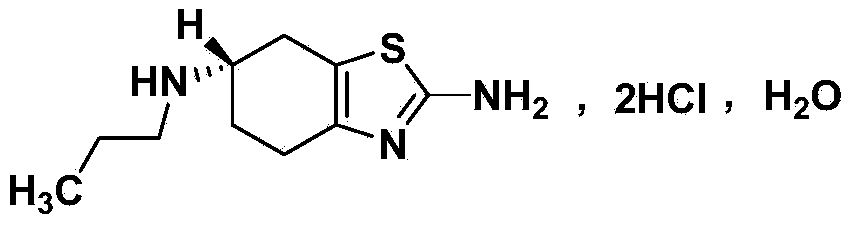
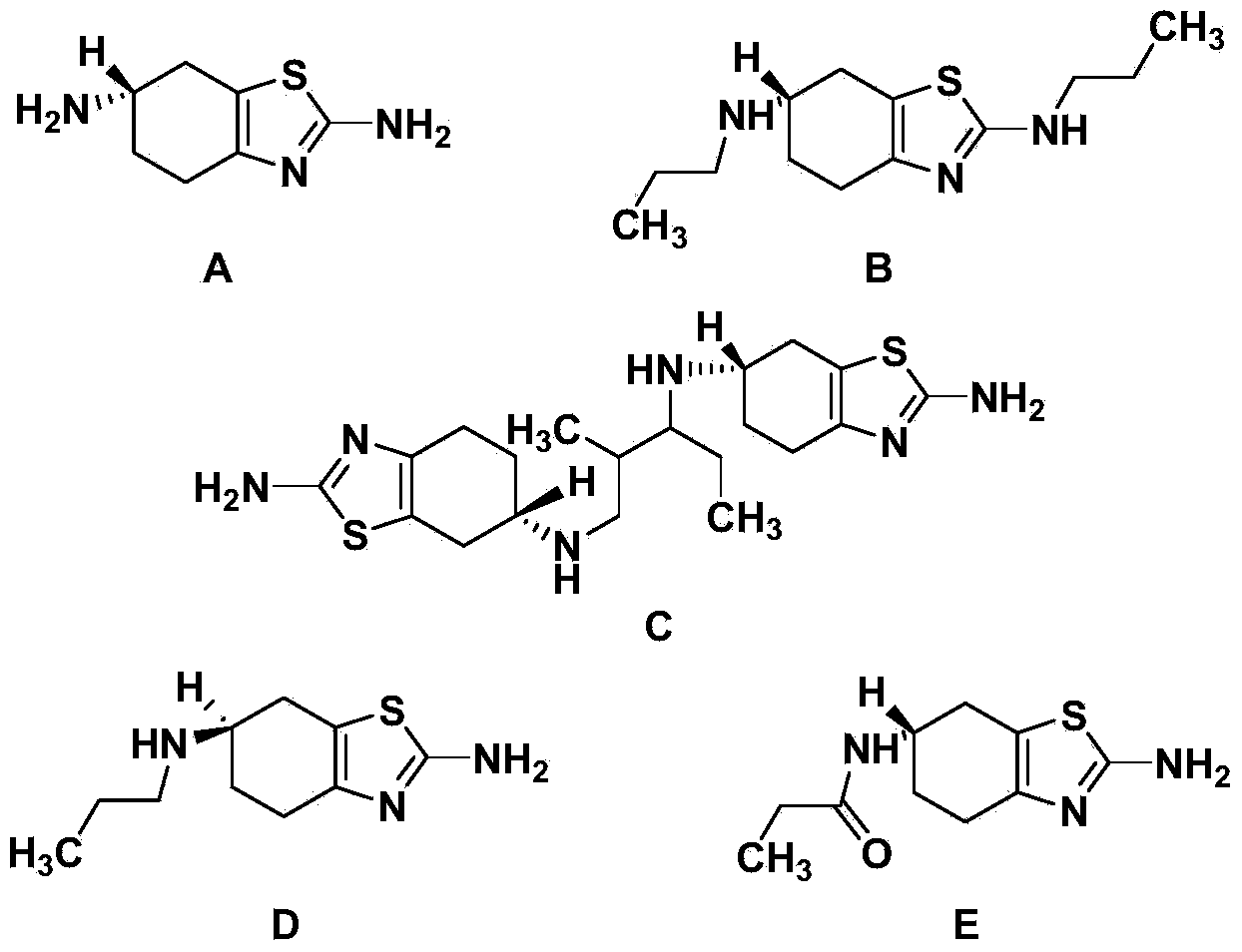
Synthetic method of pramipexole dihydrochloride related substance B
A technology for the synthesis of pramipexole hydrochloride and its synthesis method, which is applied in the field of synthesis of related substance B of pramipexole hydrochloride, which can solve the problems that the synthesis of pramipexole hydrochloride has not been reported, etc., and achieves easy-to-obtain raw materials, good repeatability, and mild reaction conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
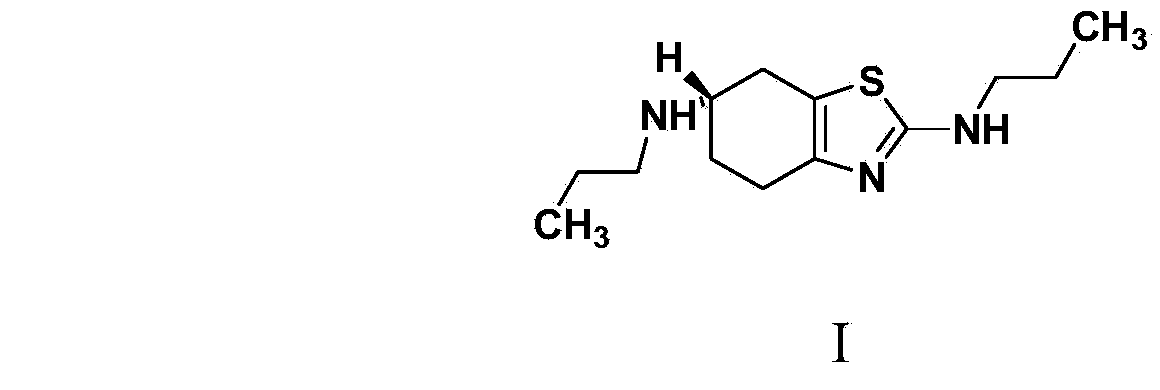
[0029] Embodiment 1: Synthesis of related substance B (i.e. compound of formula I)
[0030] (1) Synthesis of (S)-2,6-dipropionylamino-4,5,6,7-tetrahydrobenzothiazole (IV)
[0031] Suspend 1.0g of (S)-(-)-2,6-diamino-4,5,6,7-tetrahydrobenzothiazole (III) in 15ml of anhydrous tetrahydrofuran, add 1.3g of triethylamine, and A mixture of 1.2 g of propionyl chloride and 5 ml of anhydrous tetrahydrofuran was added dropwise with stirring, and stirred at room temperature for 3.5 hours. After the reaction was completed, add 10ml of water to quench the reaction, distill THF under reduced pressure, adjust the pH to 9-10 with 25% NaOH, stir for 30min, and precipitate a solid, filter, wash the filter cake with water to 7-8, and dry to obtain 1.23g of white solid , proceed directly to the next reaction.
[0032] (2): Synthesis of (S)-2,6-dipropylamino-4,5,6,7-tetrahydrobenzothiazole (I)
[0033] Suspend 1.0 g of compound (IV) in 30 ml of anhydrous tetrahydrofuran, add 0.8 g of lithium al...
Embodiment 2
[0038] Embodiment 2: Synthesis of related substance B (i.e. compound of formula I)
[0039] (1) Synthesis of (S)-2,6-dipropionylamino-4,5,6,7-tetrahydrobenzothiazole (IV)
[0040] Suspend 2.3g of (S)-(-)-2,6-diamino-4,5,6,7-tetrahydrobenzothiazole (III) in 35ml of anhydrous tetrahydrofuran, add 3.0g of triethylamine, and A mixture of 2.5 g of propionyl chloride and 12 ml of anhydrous tetrahydrofuran was added dropwise with stirring, and stirred at room temperature for 3.5 hours. After the reaction was completed, add 25ml of water to quench the reaction, distill off THF under reduced pressure, adjust the pH to 9-10 with 25% NaOH, stir for 30min, precipitate a solid, filter, wash the filter cake with water to 7-8, and obtain 2.67g of white solid after drying , proceed directly to the next reaction.
[0041] (2): Synthesis of (S)-2,6-dipropylamino-4,5,6,7-tetrahydrobenzothiazole (I)
[0042] Suspend 1.5g of compound (IV) in 45ml of anhydrous tetrahydrofuran, add 1.53g of lithi...
Embodiment 3
[0043] Embodiment 3: Synthesis of related substance B (i.e. compound of formula I)
[0044] (1) Synthesis of (S)-2,6-dipropionylamino-4,5,6,7-tetrahydrobenzothiazole (IV)
[0045] Suspend 1.3g of (S)-(-)-2,6-diamino-4,5,6,7-tetrahydrobenzothiazole (III) in 20ml of anhydrous tetrahydrofuran, add 2.2g of triethylamine, and A mixture of 1.8 g of propionyl chloride and 6.5 ml of anhydrous tetrahydrofuran was added dropwise with stirring, and stirred at room temperature for 3.5 hours. After the reaction was completed, add 13ml of water to quench the reaction, distill off THF under reduced pressure, adjust the pH to 9-10 with 25% NaOH, stir for 30min, precipitate a solid, filter, wash the filter cake with water to 7-8, and obtain 1.61g of white solid after drying , proceed directly to the next reaction.
[0046] (2): Synthesis of (S)-2,6-dipropylamino-4,5,6,7-tetrahydrobenzothiazole (I)
[0047] Suspend 1.0 g of compound (IV) in 30 ml of anhydrous tetrahydrofuran, add 0.68 g of l...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com