Method for controlling mass of related substance of pramipexole dihydrochloride tablet
A technology of pramipexole hydrochloride and pramipexole tablets, which is applied in the field of drug testing and can solve problems such as interference
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0183] Chromatographic conditions:
[0184] Use octadecylsilane bonded silica gel as filler (Shiseido CAPCELL PAK C18MGⅡ150mm × 4.6mm, 5μm);
[0185] Mobile phase A is ammonium formate buffer (pH5.0), and mobile phase B is methanol;
[0186] The flow rate is 1.0ml / min;
[0187] Column temperature 35°C;
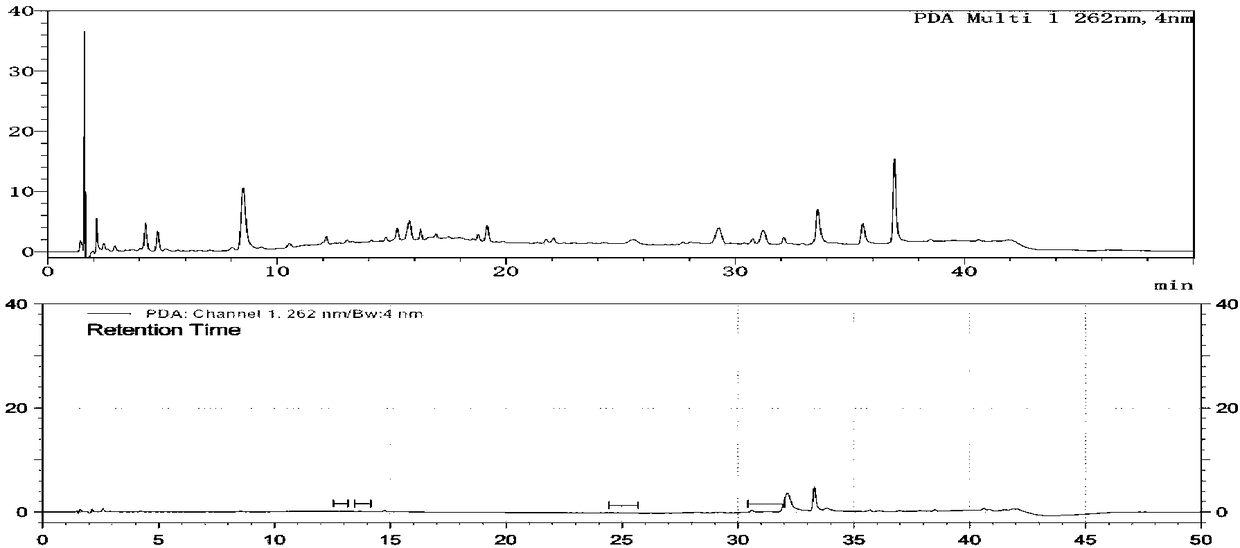
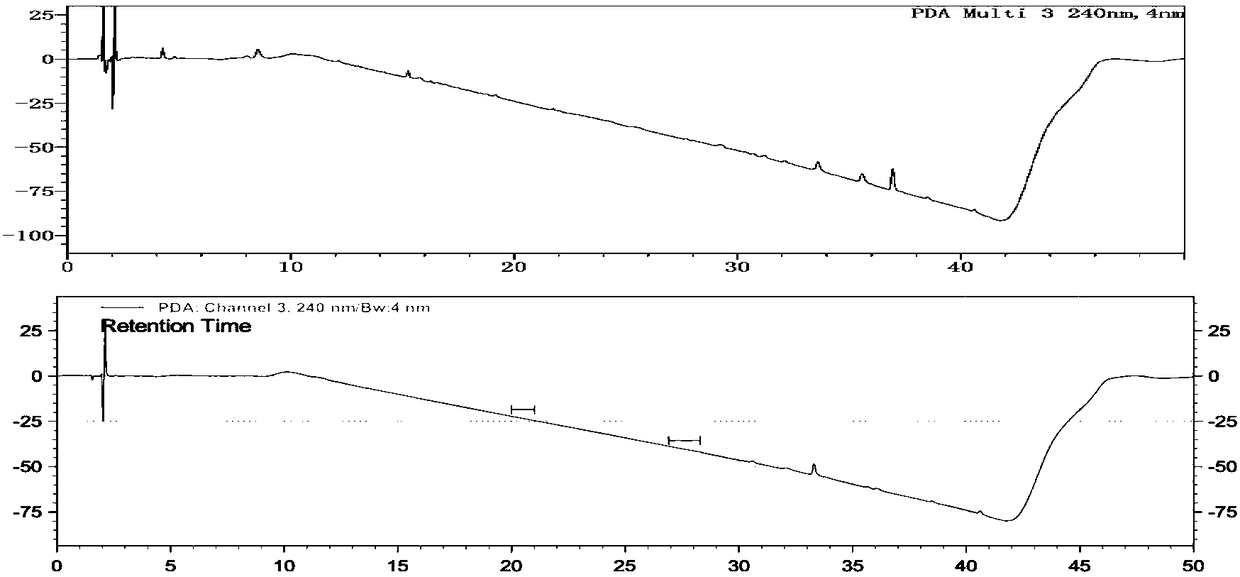
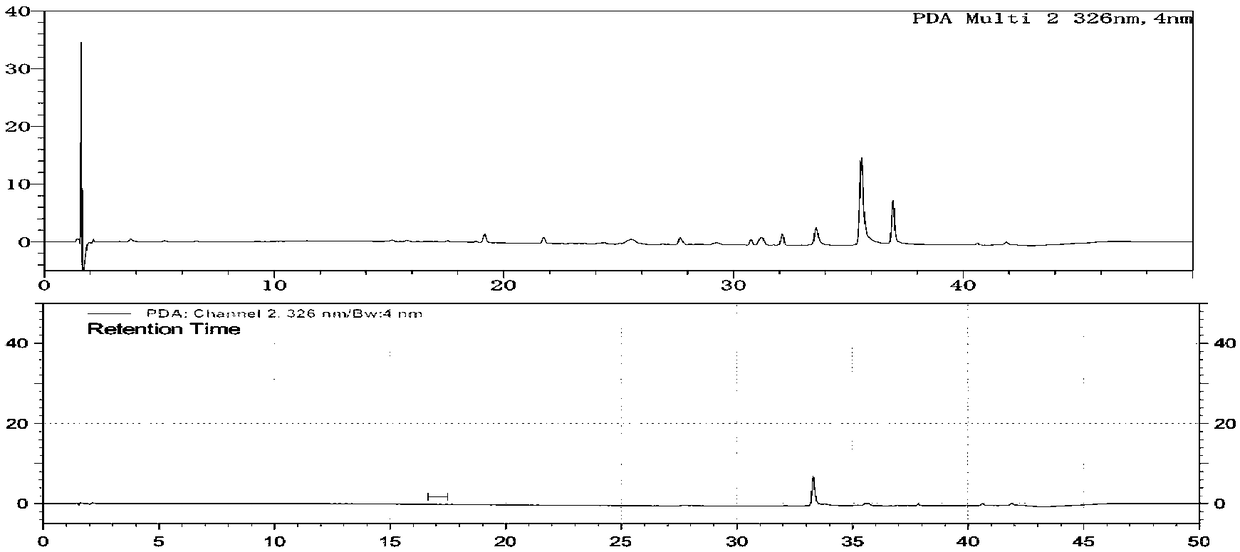
[0188] Using an array diode detector for detection, the detection wavelengths are 240nm, 262nm and 326nm;
[0189] The elution gradient is:
[0190] time (min)
Mobile phase A (V / V%)
Mobile phase B (V / V%)
0
99
1
5
99
1
38
44
56
40
99
1
50
99
1
[0191] The separation degree of each component peak in the reference solution should meet the requirements; another about 10 mg of pramipexole hydrochloride crude drug was placed in a 50 ml measuring bottle, dissolved with mobile phase and diluted to the scale, shaken, measured 10 ml and transferred to a non-glass (Quartz or transparent ...
Embodiment 2
[0204] Chromatographic conditions:
[0205] Use octadecylsilane bonded silica gel as filler (Shiseido CAPCELL PAK C18MGⅡ150mm × 4.6mm, 5μm);
[0206] Mobile phase A is ammonium formate buffer (pH4.8), and mobile phase B is methanol;
[0207] The flow rate is 1.1ml / min;
[0208] Column temperature 30°C;
[0209] Using an array diode detector for detection, the detection wavelengths are 240nm, 262nm and 326nm;
[0210] The elution gradient is:
[0211] time (min)
Mobile phase A (V / V%)
Mobile phase B (V / V%)
0
99
1
5
99
1
38
44
56
40
99
1
50
99
1
[0212] The separation degree of each component peak in the reference solution should meet the requirements; another about 10 mg of pramipexole hydrochloride crude drug was placed in a 50 ml measuring bottle, dissolved with mobile phase and diluted to the scale, shaken, measured 10 ml and transferred to a non-glass (Quartz or transparent ...
Embodiment 3
[0225] Chromatographic conditions:
[0226] Use octadecylsilane bonded silica gel as filler (Shiseido CAPCELL PAK C18MGⅡ150mm × 4.6mm, 5μm);
[0227] Mobile phase A is ammonium formate buffer (pH5.0), and mobile phase B is methanol;
[0228] The flow rate is 0.9ml / min;
[0229] Column temperature 30°C;
[0230] Using an array diode detector for detection, the detection wavelengths are 240nm, 262nm and 326nm;
[0231] The elution gradient is:
[0232] time (min)
Mobile phase A (V / V%)
Mobile phase B (V / V%)
0
99
1
5
99
1
38
44
56
40
99
1
50
99
1
[0233] The separation degree of each component peak in the reference solution should meet the requirements; another about 10 mg of pramipexole hydrochloride crude drug was placed in a 50 ml measuring bottle, dissolved with mobile phase and diluted to the scale, shaken, measured 10 ml and transferred to a non-glass (Quartz or transparent ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com