Indometacin sustained release tablet, preparation method thereof, as well as release controlling method and standard
A technology of indomethacin sustained-release tablets and indomethacin, which is applied in the field of medicine, can solve problems such as the direct preparation process of indomethacin sustained-release tablet powder and the method and standard of controlled release, and achieve production The process is simple and controllable, the standard method is accurate, and the effect of improving clinical efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0203] Preparation:
[0204] (1) Take the following components by weight:
[0205] components
unit (g)
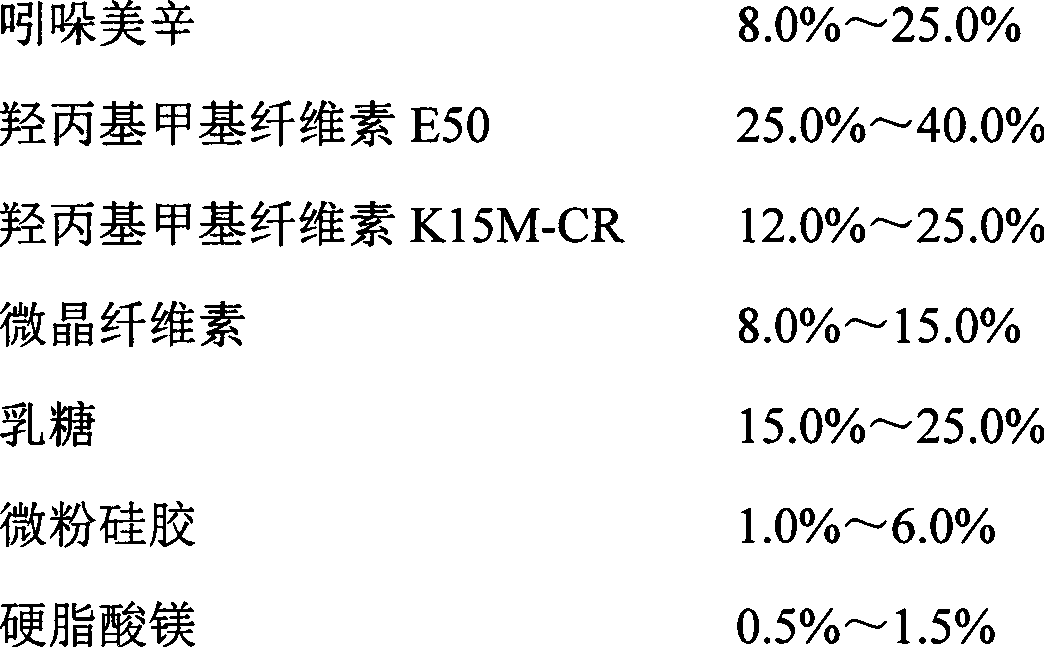
Indomethacin
198
Hydroxypropyl Methyl Cellulose E50
660
Hydroxypropyl Methyl Cellulose K15M-CR
550
217.8
541.2
Micropowder silica gel
22
11
production
10000 pieces
[0206] (2) Pass the prescribed amount of indomethacin, hydroxypropyl methylcellulose K15M-CR and micropowdered silica gel through a 100-mesh sieve for 3 times, mix for 10 minutes, and set aside;
[0207] (3) Pass the hydroxypropyl methylcellulose E50 of the prescribed amount through an 80-mesh sieve once, add the materials in the above (2), mix for 9 minutes, and set aside;
[0208] (4) Pass the prescribed amount of microcrystalline cellulose and lactose through an 80-mesh sieve once, add the materials in the above (3), mix for 6 minutes, and s...
Embodiment 2
[0213] Preparation:
[0214] (1) Take the following components by weight:
[0215] components
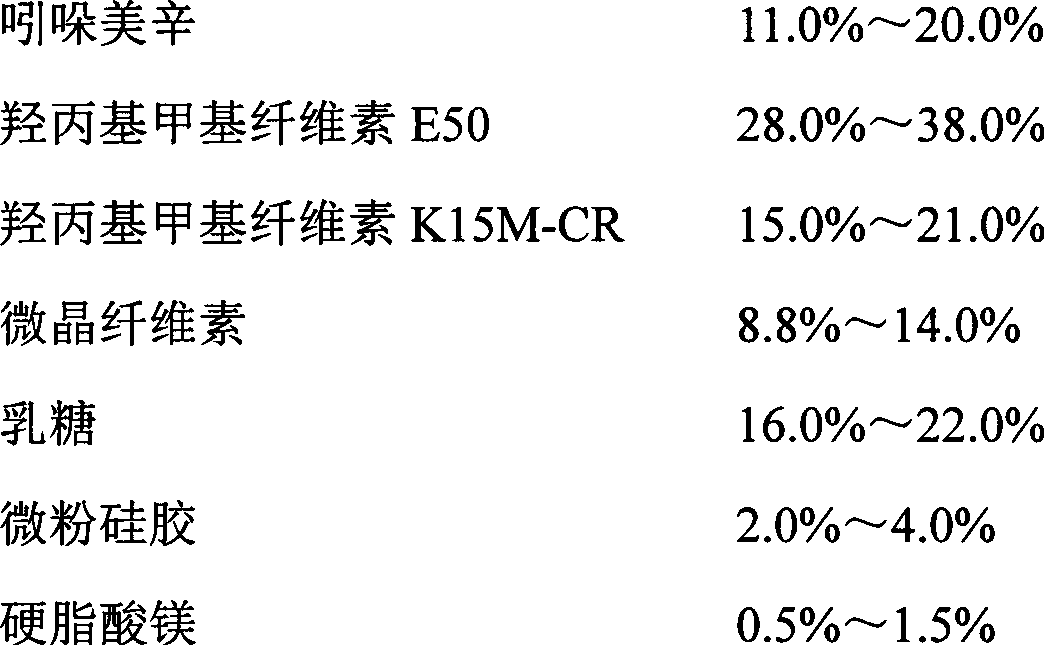
unit (g)
Indomethacin
528
Hydroxypropyl Methyl Cellulose E50
550
Hydroxypropyl Methyl Cellulose K15M-CR
286
242
440
Micropowder silica gel
121
33
production
10000 pieces
[0216] (2) Pass the prescribed amount of indomethacin, hydroxypropyl methylcellulose K15M-CR and micropowdered silica gel through a 100-mesh sieve for 3 times, mix for 6 minutes, and set aside;
[0217] (3) Pass the hydroxypropyl methylcellulose E50 of the prescription amount through an 80-mesh sieve once, add the materials in the above (2), mix for 6 minutes, and set aside;
[0218] (4) Pass the prescribed amount of microcrystalline cellulose and lactose through an 80-mesh sieve once, add the materials in the above (3), mix for 10 minutes, and se...
Embodiment 3
[0223] Preparation:
[0224] (1) Take the following components by weight:
[0225] components
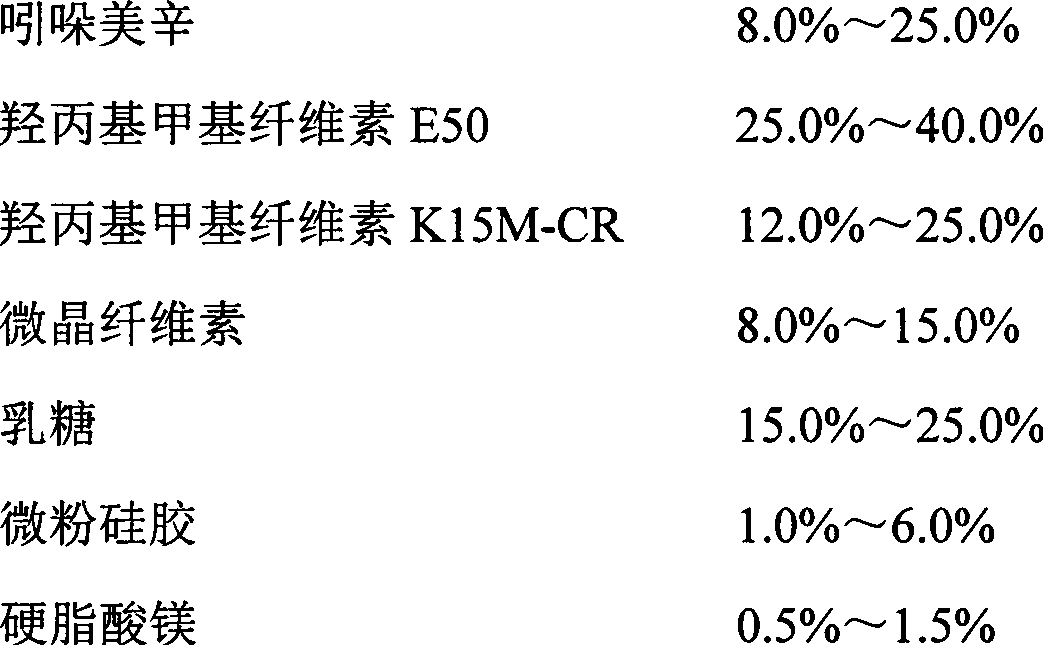
unit (g)
Indomethacin
396
Hydroxypropyl Methyl Cellulose E50
660
Hydroxypropyl Methyl Cellulose K15M-CR
462
microcrystalline cellulose
242
lactose
330
Micropowder silica gel
77
Magnesium stearate
33
production
10000 pieces
[0226] (2) Pass the prescribed amount of indomethacin, hydroxypropyl methylcellulose K15M-CR and micropowdered silica gel through a 100-mesh sieve for 3 times, mix for 15 minutes, and set aside;
[0227] (3) Pass the hydroxypropyl methylcellulose E50 of the prescription amount through an 80-mesh sieve once, add the materials in the above (2), mix for 8 minutes, and set aside;
[0228] (4) Pass the prescribed amount of microcrystalline cellulose and lactose through an 80-mesh sieve once, add the materials in the above (3), mix for 8 minutes, and set...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com