Diclofenac sodium dual-release enteric-coated preparation, as well as preparation method and control method thereof
A technology of diclofenac sodium and enteric-coated preparations, which is applied in the field of medicine, can solve problems such as hindering clinical application, many side effects, and short biological half-life, and achieve the effects of reducing gastrointestinal tract irritation and the number of medications, stable and reliable quality, and improving production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
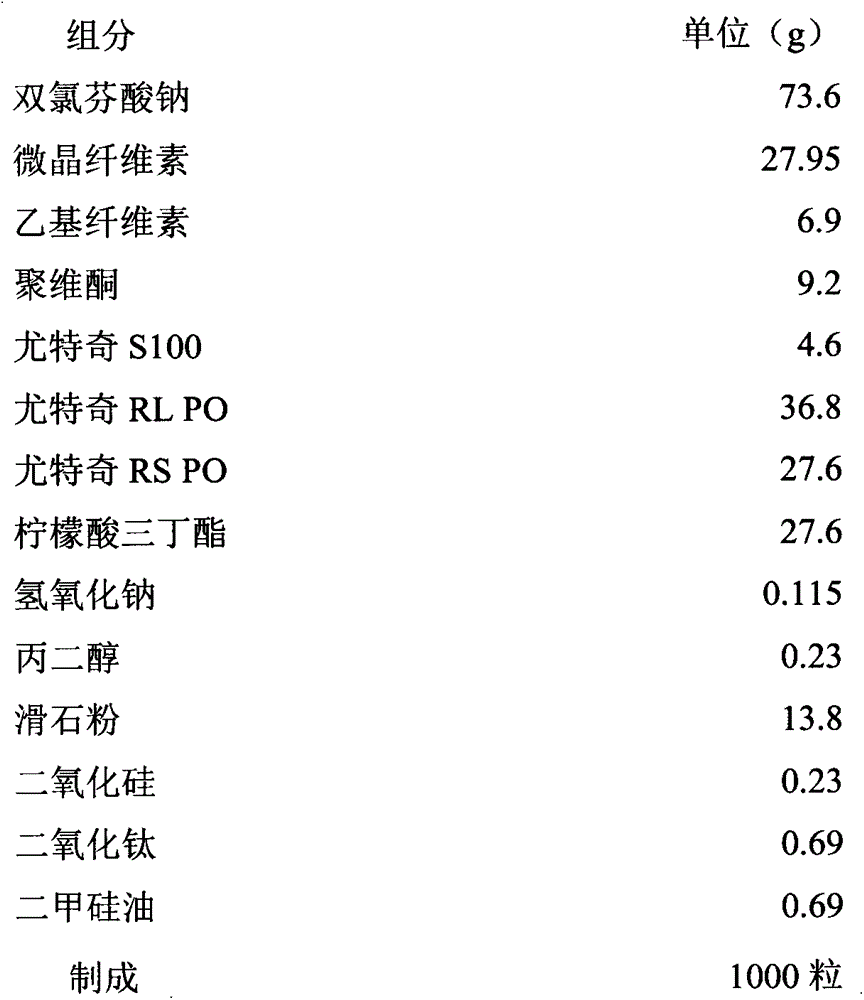
[0131]
[0132] 1) Preparation method:
[0133] (1) Take the components by the above weight;
[0134] (2) Mix 73.6g diclofenac sodium, 43.08g microcrystalline cellulose, 9.2g ethyl cellulose and 16.1g povidone evenly, use distilled water as a wetting agent to make a soft material, put the prepared soft material into the extruder Out of the spheronizing granulator, the prepared pills are dried and set aside;
[0135] (3) 0.161g sodium hydroxide is configured into a 5% aqueous solution for subsequent use;
[0136] (4) Slowly add 9.2g of Eudragit S100 to 4 times the amount of distilled water, add 6.9g of talc powder and 0.23g of titanium dioxide, stir for 28 minutes, add the sodium hydroxide solution in the above (3), and then add 1.15g Propylene glycol, stir evenly, add 0.23g of simethicone dropwise, and set aside;
[0137] (5) Mix Eudragit 27.6g RL PO with Eudragit 13.8g RS PO, slowly add to 20 times the amount of 95% ethanol, add 13.8g of talcum powder and 0.46g of titan...
Embodiment 2
[0142]
[0143] 1) Preparation method:
[0144] (1) Take the components by the above weight;
[0145] (2) Mix 73.6g diclofenac sodium, 37.7g microcrystalline cellulose, 11.5g ethyl cellulose and 18.4g povidone evenly, use distilled water as a wetting agent to make a soft material, put the prepared soft material into an extruder Out of the spheronizing granulator, the prepared pills are dried and set aside;
[0146] (3) 0.21g sodium hydroxide is configured into a 4% aqueous solution for subsequent use;
[0147] (4) Slowly add 6.9g of Eudragit S100 to 3 times the amount of distilled water, add 6.9g of talc powder and 0.23g of titanium dioxide, stir for 20 minutes, add the sodium hydroxide solution in the above (3), and then add 1.84g Propylene glycol, stir evenly, add 0.23g of simethicone dropwise, and set aside;
[0148] (5) Mix Eudragit 20.7g RL PO with Eudragit 18.4g RS PO, slowly add to 18 times the amount of 95% ethanol, add 11.5g of talcum powder and 0.46g of titaniu...
Embodiment 3
[0153]
[0154] 1) Preparation method:
[0155] (1) take components by weight;
[0156] (2) Mix 73.6g diclofenac sodium, 48.76g microcrystalline cellulose, 11.5g ethyl cellulose and 23g povidone evenly, use distilled water as a wetting agent to make a soft material, put the prepared soft material into extrusion In the spheronizing granulator, the prepared pills are dried and set aside;
[0157] (3) 0.23g sodium hydroxide is configured into 8% aqueous solution, for subsequent use;
[0158] (4) Slowly add 11.5g of Eudragit S100 to 5 times the amount of distilled water, add 16.1g of talc powder and 0.23g of titanium dioxide, stir for 30 minutes, add the sodium hydroxide solution in the above (3), and then add 2.3g Propylene glycol, stir evenly, add 0.23g of simethicone dropwise, and set aside;
[0159](5) Mix Eudragit 6.9g RL PO with Eudragit 6.9g RS PO, slowly add to 10 times the amount of 95% ethanol, add 16.1g of talcum powder and 0.46g of titanium dioxide, stir for 25 m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| fluorescence | aaaaa | aaaaa |
| fluorescence | aaaaa | aaaaa |
| fluorescence | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com