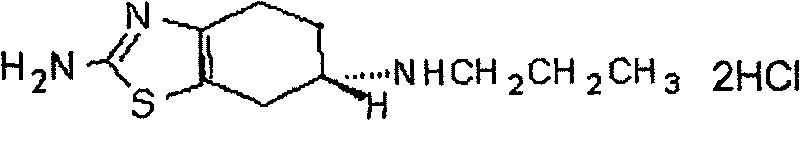
Preparation of pramipexole
A technology of pramipexole hydrochloride and benzothiazole, which is applied in the direction of organic chemistry, can solve the problems of low reaction safety and difficult preparation of raw materials, and achieve the effects of mild reaction conditions, improved reaction safety, and easy control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
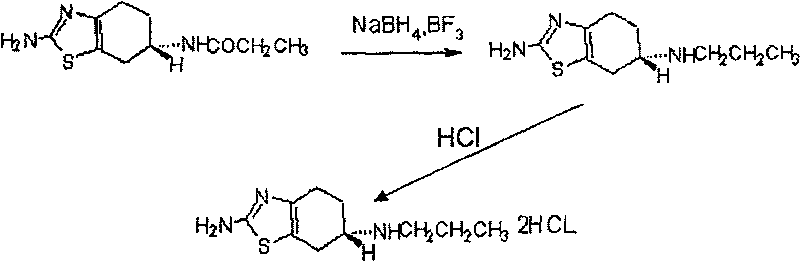
[0009] The preparation method of pramipexole hydrochloride provided by the invention can be represented by following reaction equation 1:
[0010]
[0011] Reaction Equation 1
[0012] In the first step reaction of reaction equation 1, the (-) 2-amino-6-propionylamino-4,5,6,7-tetrahydrobenzothiazole, NaBH 4 and BF 3 The molar ratio is 1:4:6-1:10:13, preferably 1:6:8-1:9:11.
[0013] Described tetrahydrofuran (THF) is used as solvent, and its volume consumption is NaBH 4 4-10 times the weight (volume / weight).
[0014] The inert gas refers to any gas that does not react chemically with reactants or products, such as nitrogen, one or more of the gases of group zero in the periodic table, preferably nitrogen.
[0015] During the reaction, (-) 2-amino-6-propionylamino-4,5,6,7-tetrahydrobenzothiazole, NaBH 4 and BF 3 The order of addition is preferably, first NaBH 4 Added into tetrahydrofuran, and then added (-) 2-amino-6-propionylamino-4,5,6,7-tetrahydrobenzothiazole and ...
Embodiment 1
[0022] This embodiment is used to illustrate the preparation method of pramipexole hydrochloride of the present invention.
[0023] (1) Preparation of (S)-(-)2-amino-6-propylamino-4,5,6,7-tetrahydrobenzothiazole
[0024] 0.325 M NaBH 4 Add to the three-necked flask that fills 60 milliliters of anhydrous THF, pass into N 2 Under gas protection, at a temperature of 2°C and under constant stirring, add 0.05 mole (-) 2-amino-6-propionylamino-4,5,6,7-tetrahydrobenzothiazole, mix well and then add 100 mL of 5 mol / L BF 3 Diethyl ether solution (0.5 mole BF 3 ). Then the temperature was raised to 10° C., and reacted for 1.5 hours under constant stirring, and then the reaction mixture was heated to 45° C., reacted for 4.5 hours under constant stirring, and then cooled at 2° C. for 18 minutes.
[0025]To the above reacted mixture was added 200 ml of 20% hydrochloric acid solution, and then THF was evaporated. Dissolve the residue in 600 ml of water, adjust the pH to 12.5 with 0.5 ...
Embodiment 2
[0029] This embodiment is used to illustrate the preparation method of pramipexole hydrochloride of the present invention.
[0030] (1) Preparation of (S)-(-)2-amino-6-propylamino-4,5,6,7-tetrahydrobenzothiazole
[0031] 0.40 M NaBH 4 Add to the three-necked flask that fills 120 milliliters of anhydrous THF, pass into N 2 Under gas protection, at a temperature of 4°C and under constant stirring, add 0.05 mole (-) 2-amino-6-propionylamino-4,5,6,7-tetrahydrobenzothiazole, mix well and then add 70 mL of 6 mol / L BF 3 Ether solution (0.42 mole BF 3 ). Then the temperature was raised to 30° C. and reacted for 2.5 hours under constant stirring, then the reaction mixture was heated to 55° C., reacted for 2.5 hours under constant stirring, and then cooled at 5° C. for 10 minutes.
[0032] To the above reacted mixture was added 350 ml of 22% hydrochloric acid solution, and THF was evaporated. The residue was then dissolved in 600 ml of water, adjusted to pH 10.5 with 0.5 mol / L NaO...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com