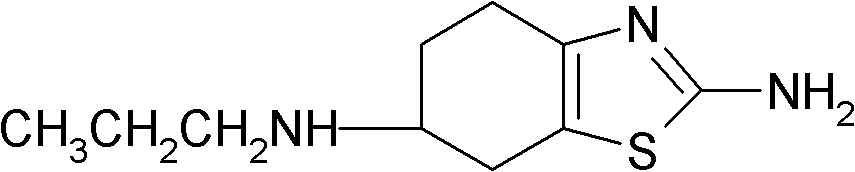
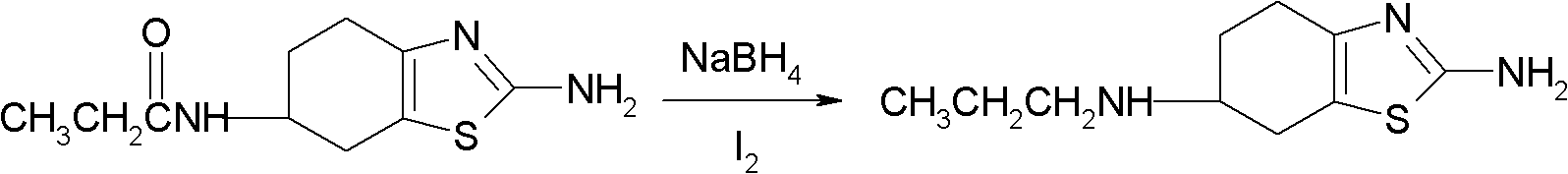Preparation method of 2-amino-6-propylamino-4,5,6,7-tetrahydrobenzothiazole
A technology of benzothiazole and propylamino, applied in the field of medicine, can solve the problems of low reaction safety, unfavorable, difficult preparation, etc., and achieves the effects of simple raw materials, easy availability of raw materials, and improved reaction safety.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] 0.18 M NaBH 4 Add it into a three-necked reaction flask containing 50 ml of anhydrous tetrahydrofuran, pass through nitrogen protection, and add 0.02 moles of 2-amino-6-propionylamino-4,5 under the condition of constant stirring at 0-5°C, 6,7-Tetrahydrobenzothiazole, after mixing evenly, slowly drop into 100 ml of 0.08 mole I 2 THF solution (0.08 mol I 2 ), the time is 3-4 hours. Then, the temperature was controlled at 0-5° C. and the reaction was continuously stirred for 10 hours, and the reaction mixture was heated to 50° C. for 12 hours. Then cool under an ice bath.
[0022] Add 350 milliliters of 10% hydrochloric acid solution to the above-mentioned reacted mixture, adjust the pH value to 9-10 with 20% NaOH solution, extract in portions with ethyl acetate, add anhydrous sodium sulfate to the extract to dry, The solvent was evaporated to obtain a solid product, which was dried in vacuo to obtain 2.54 g of 2-amino-6-propylamino-4,5,6,7-tetrahydrobenzothiazole, and...
Embodiment 2
[0025] 0.18 M NaBH 4 Add it into a three-necked reaction flask containing 50 ml of anhydrous tetrahydrofuran, pass through nitrogen protection, and add 0.02 moles of 2-amino-6-propionylamino-4,5 under the condition of constant stirring at 0-5°C, 6,7-Tetrahydrobenzothiazole, after mixing evenly, slowly drop into 100 milliliters of 0.08 moles of I 2 Tetrahydrofuran, ether solution (0.08 moles I 2 , the volume ratio of tetrahydrofuran to diethyl ether is 1:1), reacted at 0-5°C for 12 hours under constant stirring, then heated the reaction mixture to 35°C for 12 hours, and then cooled it in an ice bath.
[0026] Add 360 milliliters of 10% hydrochloric acid solution to the above-mentioned reacted mixture, adjust the pH value to 9-10 with 20% NaOH solution, extract in portions with ethyl acetate, add anhydrous sodium sulfate to the extract to dry, The solvent was evaporated to obtain a solid product, which was dried in vacuo to obtain 2.75 g of 2-amino-6-propylamino-4,5,6,7-tetrah...
Embodiment 3
[0029] 0.04 M NaBH 4 Add it into a three-necked reaction flask containing 50 ml of anhydrous tetrahydrofuran, pass through nitrogen protection, and add 0.02 moles of 2-amino-6-propionylamino-4,5 under the condition of constant stirring at 0-5°C, 6,7-Tetrahydrobenzothiazole, after mixing evenly, slowly drop into 100 milliliters of 0.16 moles of I 2 Tetrahydrofuran, ether solution (0.16 moles I 2 , the volume ratio of tetrahydrofuran to diethyl ether is 1:4), reacted at 0-5°C for 12 hours under constant stirring, then heated the reaction mixture to 40°C for 20 hours, and then cooled it in an ice bath.
[0030] Add 360 milliliters of 10% hydrochloric acid solution to the above-mentioned reacted mixture, adjust the pH value to 9-10 with 20% NaOH solution, extract in portions with ethyl acetate, add anhydrous sodium sulfate to the extract to dry, The solvent was evaporated to obtain a solid product, which was dried in vacuo to obtain 2.33 g of 2-amino-6-propylamino-4,5,6,7-tetrah...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com