Device and method for synthesizing bi(trichloromethyl) carbonic ester
A technology of trichloromethyl group and synthesis device, applied in the field of dicarbonate synthesis device, can solve the problems of high environmental pollution pressure, high storage and transportation pressure, increased consumption of sodium hydroxide and water, etc., so as to improve product yield. efficiency and conversion of dimethyl carbonate, reducing storage and environmental pressures, and increasing reaction safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
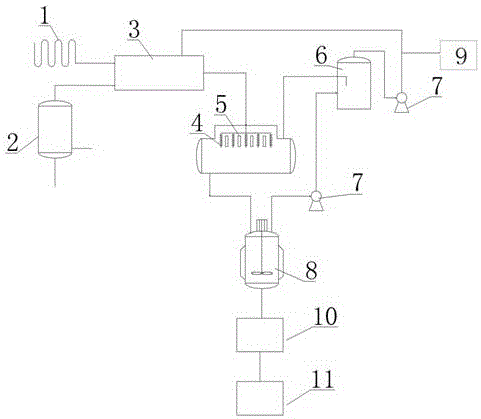
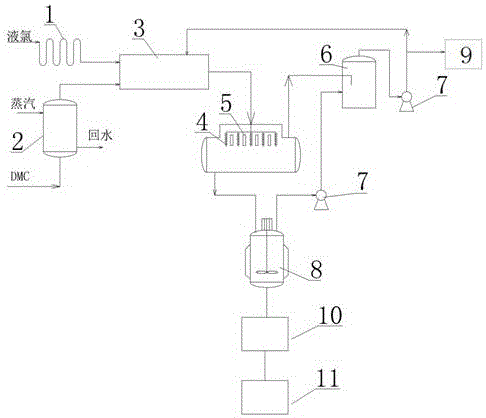
[0035] Embodiment 1 Utilize the synthetic device of above-mentioned two (trichloromethyl) carbonates to synthesize two (trichloromethyl) carbonates
[0036] The steps are as follows (process flow chart as figure 2 shown):
[0037] (1) Water vapor and dimethyl carbonate are passed into dimethyl carbonate vaporizer (solvent 15m 3 ), the dimethyl carbonate is vaporized, and the temperature is controlled at 90-93° C.; the liquid chlorine is passed into the liquid chlorine vaporizer, and the liquid chlorine is gasified into chlorine gas;
[0038] (2) Chlorine and vaporized dimethyl carbonate enter the heat exchanger in a molar ratio of 6:1. After heat exchange, a mixed gas phase is obtained, and the temperature of the mixed gas phase is controlled at 90-93° C.;
[0039] (3) The mixed gas phase is sprayed from the top into a column-and-tube reactor (capacity 6m) that is vertically filled with a solid catalyst (ceramics as a carrier, and the catalyst is a mixture of copper chlorid...
Embodiment 2
[0042] Embodiment 2 Utilize the synthetic device of above-mentioned bis(trichloromethyl)carbonate to synthesize bis(trichloromethyl)carbonate
[0043] Proceed as follows:
[0044] (1) Water vapor and dimethyl carbonate are passed into dimethyl carbonate vaporizer (solvent 15m 3 ), the dimethyl carbonate is vaporized, and the temperature is controlled at 93 to 95° C.; the liquid chlorine is passed into the liquid chlorine vaporizer, and the liquid chlorine is gasified into chlorine gas;
[0045] (2) Chlorine and vaporized dimethyl carbonate enter the heat exchanger in a molar ratio of 6:1, and a mixed gas phase is obtained after heat exchange, and the temperature of the mixed gas phase is controlled at 93 to 95°C;
[0046] (3) The mixed gas phase is sprayed from the top into a column-and-tube reactor (capacity 6m) that is vertically filled with a solid catalyst (ceramics as a carrier, and the catalyst is a mixture of copper chloride and zinc chloride) through a mixed gas phase...
Embodiment 3
[0050] Embodiment 3 Utilize the synthetic device of above-mentioned bis(trichloromethyl)carbonate to synthesize bis(trichloromethyl)carbonate
[0051] Proceed as follows:
[0052] (1) Water vapor and dimethyl carbonate are passed into dimethyl carbonate vaporizer (solvent 15m 3 ), the dimethyl carbonate is vaporized, and the temperature is controlled at 95 to 98° C.; the liquid chlorine is passed into the liquid chlorine vaporizer, and the liquid chlorine is gasified into chlorine gas;
[0053] (2) Chlorine and vaporized dimethyl carbonate enter the heat exchanger in a molar ratio of 6:1, and a mixed gas phase is obtained after heat exchange, and the temperature of the mixed gas phase is controlled at 95-98°C;
[0054] (3) The mixed gas phase is sprayed from the top into a column-and-tube reactor (capacity 6m) that is vertically filled with a solid catalyst (ceramics as a carrier, and the catalyst is a mixture of copper chloride and zinc chloride) through a mixed gas phase di...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com