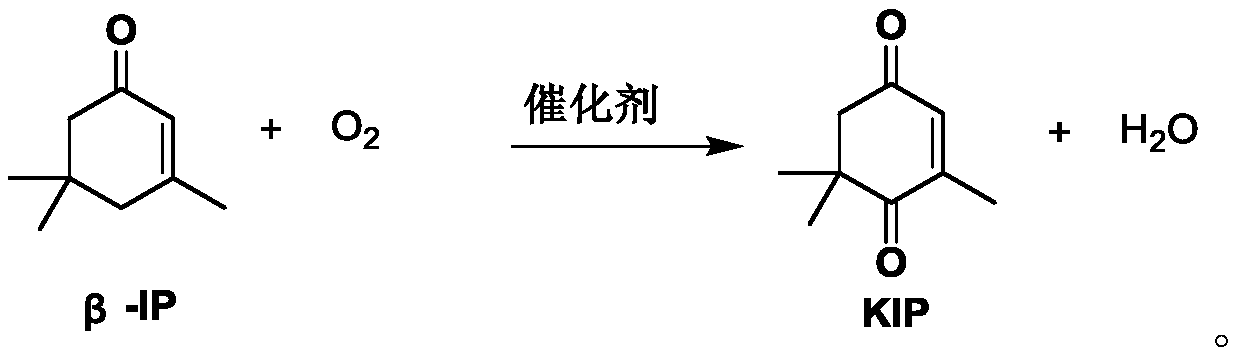
Method for continuously synthesizing 4-oxoisophorone in microchannel reactor
A microchannel reactor, oxoisophorone technology, applied in chemical instruments and methods, chemical/physics/physicochemical reactors, preparation of organic compounds, etc., can solve the problem of lack of industrialization and expensive transition metal catalysts , the problem of high cost of the reaction process, to reduce the abnormal working conditions that block the microchannel reactor, improve the experimental stability and operability, and improve the stability and operability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Material preparation:
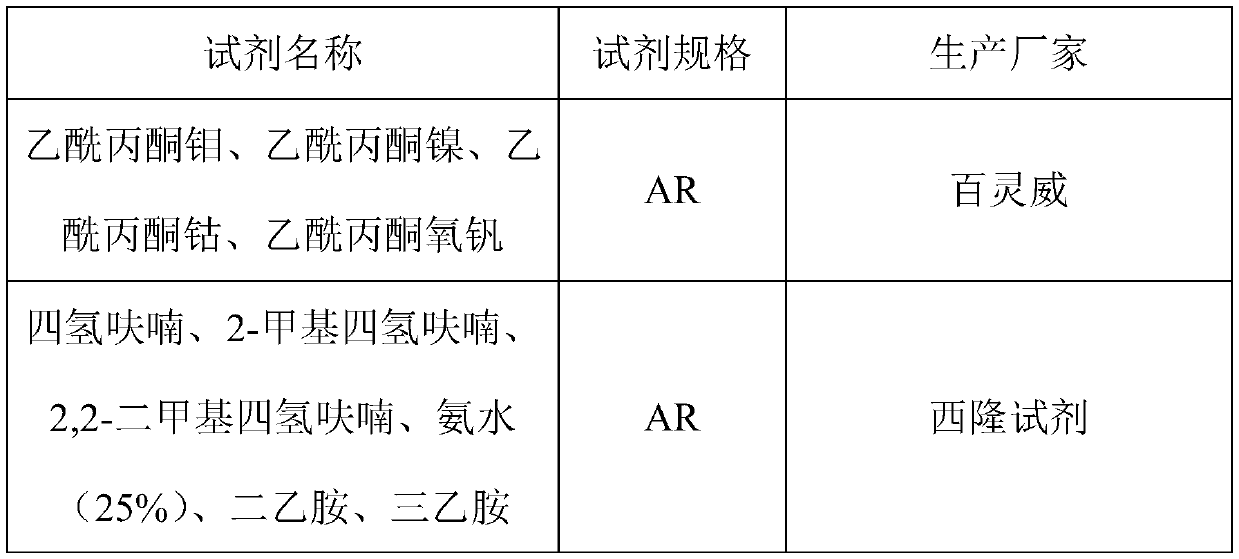
[0048] Weigh 2.00g of molybdenum acetylacetonate, 8.00g of ammonia water (25%), and 990g of tetrahydrofuran, and mix them uniformly in a 2L glass jar; weigh 400g of β-isophorone and put them into a 500mL glass jar. Put the β-isophorone and catalyst solution into the ultrasonic generator for 30 minutes to remove the dissolved gas in the material.
[0049] System preparation:
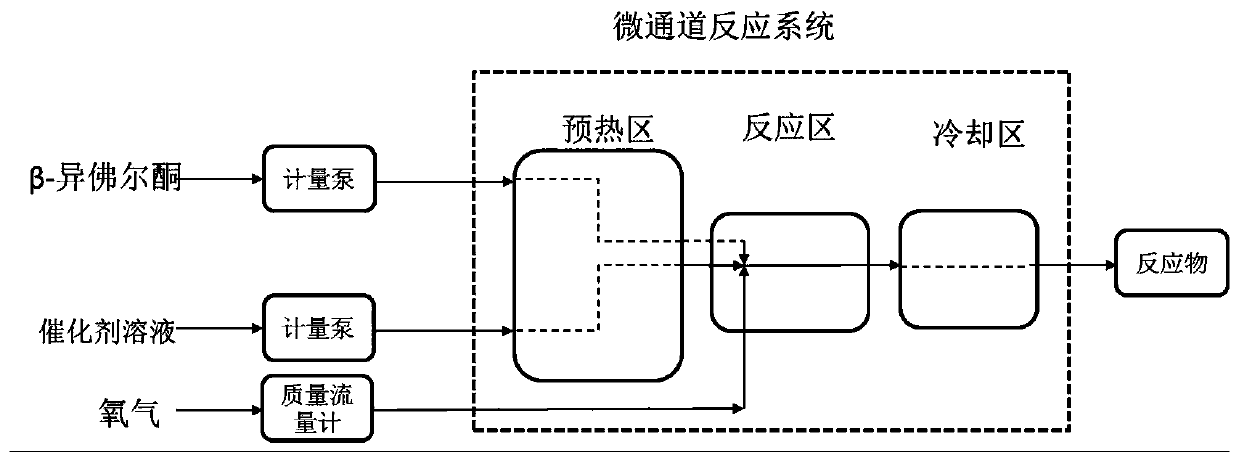
[0050] Turn on the microchannel reaction system, set the temperature of the heat transfer oil in the preheating zone to 50°C, set the temperature of the heat transfer oil in the reaction zone to 60°C, and set the temperature of the heat transfer oil in the cooling zone to 20°C, and wait for the temperature to balance.
[0051] Preparation of 4-oxoisophorone:
[0052] After the temperature of the heat transfer oil is stable, take out the β-isophorone and the catalyst solution from the ultrasonic generator, inject the catalyst solution into the microchannel system at a rate ...
Embodiment 2
[0054] Material preparation:
[0055] Weigh 2.50g of vanadyl acetylacetonate, 3.50g of triethylamine, and 994g of tetrahydrofuran, and mix them uniformly in a 2L glass jar; weigh 400g of β-isophorone into a 500mL glass jar. Put the β-isophorone and catalyst solution into the ultrasonic generator for 30 minutes to remove the dissolved gas in the material.
[0056] System preparation:
[0057] Turn on the microchannel reaction system, set the temperature of the heat transfer oil in the preheating zone to 50°C, set the temperature of the heat transfer oil in the reaction zone to 60°C, and set the temperature of the heat transfer oil in the cooling zone to 20°C, and wait for the temperature to balance.
[0058] Preparation of 4-oxoisophorone:
[0059] After the temperature of the heat transfer oil is stable, take out the β-isophorone and catalyst solution from the ultrasonic generator, inject the catalyst solution into the microchannel system at a rate of 8 g / min with an advection...
Embodiment 3
[0061] Material preparation:
[0062] Weigh 3.00g of nickel acetylacetonate, 3.00g of diethylamine, and 994g of 2-methyltetrahydrofuran, and mix them uniformly in a 2L glass jar; weigh 400g of β-isophorone into a 500mL glass jar. Put the β-isophorone and catalyst solution into the ultrasonic generator for 30 minutes to remove the dissolved gas in the material.
[0063] System preparation:
[0064] Turn on the microchannel reaction system, set the temperature of the heat transfer oil in the preheating zone to 65°C, set the temperature of the heat transfer oil in the reaction zone to 75°C, and set the temperature of the heat transfer oil in the cooling zone to 25°C, and wait for the temperature to balance.
[0065] Preparation of 4-oxoisophorone:
[0066] After the temperature of the heat transfer oil is stable, take out the β-isophorone and catalyst solution from the ultrasonic generator, inject the catalyst solution into the microchannel system at a rate of 2g / min with an ad...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com