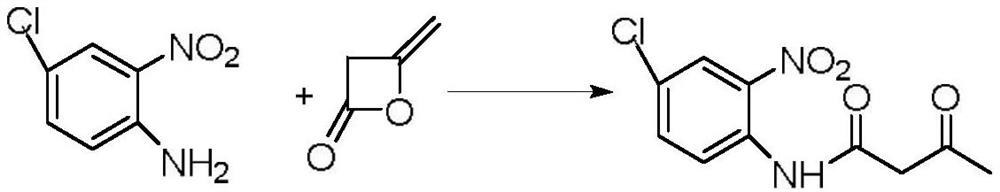
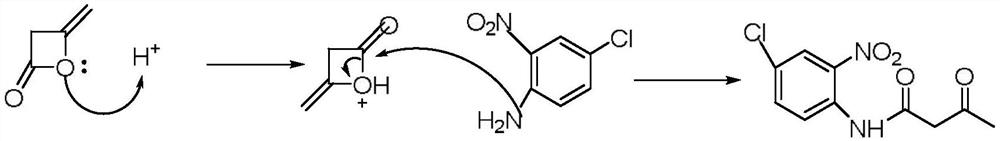
Method for preparing p-chloro-o-nitroacetoacetanilide by using packed bed reactor
A technology of nitroacetoacetanilide and packed bed reactors, which is applied in chemical instruments and methods, preparation of carboxylic acid amides, preparation of organic compounds, etc. It can solve the problems of difficult recovery and reuse of catalysts, high requirements for reaction conditions, and poor production safety To achieve the effect of improving reaction efficiency and product selectivity, improving product yield and quality, and high load
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
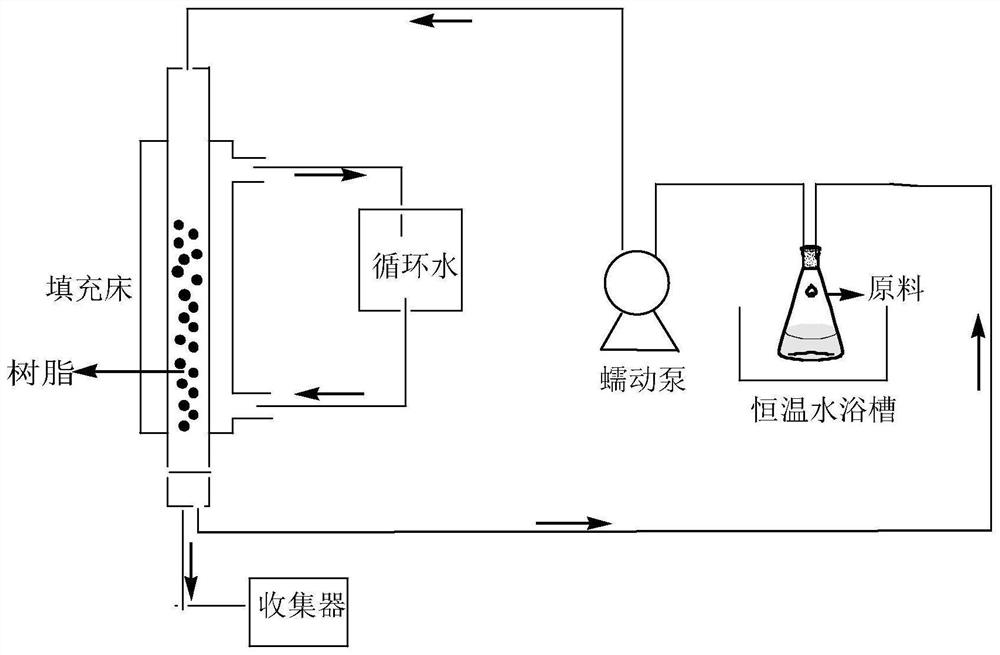
[0029] A kind of method utilizing packed bed reactor to prepare p-chloro-o-nitroacetoacetanilide, concrete steps are as follows:
[0030] (1) preparation mass fraction is 250g of p-chloro-o-nitroaniline toluene solution, according to the ratio of p-chloro-o-nitroaniline and diketene molar ratio 1:1.5, diketene is added to the above-mentioned p-chloro-o-nitroaniline toluene solution , stirring and heating until all the solids are dissolved to obtain a reaction liquid, the temperature of the heating process is controlled below 80°C; + The molar ratio is 1:0.2, and the hydrogen type ZSM-5 zeolite molecular sieve is filled in the packed bed;
[0031] (2) Turn on the heating circulation device, set the temperature of the packed bed reactor to be 80°C, use a peristaltic pump to inject the reaction solution into the reactor according to the flow rate of 25ml / min, and make the reaction solution react in the reactor;
[0032] (3) Detect the reaction solution collected in the collector...
Embodiment 2
[0034] A kind of method utilizing packed bed reactor to prepare p-chloro-o-nitroacetoacetanilide, concrete steps are as follows:
[0035] (1) preparation mass fraction is 250g of p-chloro-o-nitroaniline toluene solution, according to the ratio of p-chloro-o-nitroaniline and diketene molar ratio 1:1.5, diketene is added to the above-mentioned p-chloro-o-nitroaniline toluene solution In the process, stir and heat until the solids are all dissolved to obtain a reaction liquid, and the temperature during the heating process is controlled below 80°C; + The molar ratio is 1:0.2, the acid clay kaolin catalyst is filled in the packed bed;
[0036] (2) Turn on the heating circulation device, set the temperature of the packed bed reactor to be 80°C, use a peristaltic pump to inject the reaction solution into the reactor according to the flow rate of 25ml / min, and make the reaction solution react in the reactor;
[0037] (3) Detect the reaction solution collected in the collector, track...
Embodiment 3
[0039] A kind of method utilizing packed bed reactor to prepare p-chloro-o-nitroacetoacetanilide, concrete steps are as follows:
[0040] (1) preparation mass fraction is 250g of p-chloro-o-nitroaniline toluene solution, according to the ratio of p-chloro-o-nitroaniline and diketene molar ratio 1:1.5, diketene is added to the above-mentioned p-chloro-o-nitroaniline toluene solution In the process, stir and heat until the solids are completely dissolved to obtain a reaction solution, and the temperature during the heating process is controlled below 80°C; +Molar ratio 1:0.14, D001 acidic cation exchange resin is filled in the packed bed;
[0041] (2) Turn on the heating circulation device, set the temperature of the packed bed reactor to be 80°C, use a peristaltic pump to inject the reaction solution into the reactor according to the flow rate of 25ml / min, and make the reaction solution react in the reactor;
[0042] (3) Detect the reaction solution collected in the collector,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com