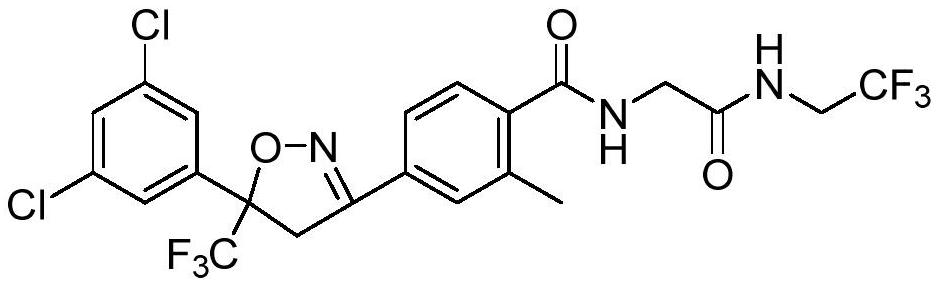
Synthetic method of fluralana
A technique for the synthesis of frellaner and a synthetic method, which is applied in the field of frellaner synthesis, can solve the problems of high economic cost, low yield, long reaction time, etc., so as to improve economic benefits, reduce production costs, and reduce reagent consumption Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
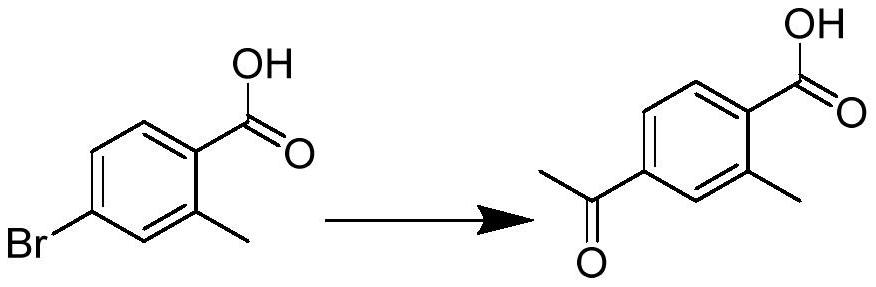
[0041] Embodiment 1: Preparation of 4-acetyl-2-methylbenzoic acid (intermediate 1)
[0042]
[0043] 4-Bromo-2-methyl-benzoic acid (compound 0) (0.277g, 1.29mmol), n-butyl vinyl ether (0.83ml, 6.42mmol), 1,3-bis(diphenylphosphine)propane (0.17g, 0.04mmol), palladium acetate (0.0023g, 0.01mmol), potassium carbonate (0.3g, 2.17mmol) were added to the bottle, 10mL of n-butanol was added, nitrogen was replaced 3 times, and the heating temperature was controlled to reflux at 90°C After 9 to 10 hours, the reaction of the raw materials is complete, stop heating, cool to room temperature, add water and concentrated hydrochloric acid to adjust the pH to 1-2, extract with ethyl acetate, wash the organic phase with water in turn, wash with saturated aqueous sodium chloride solution, and diatomaceous earth After filtration, it was dried over anhydrous sodium sulfate and spin-dried. 0.263 g of compound 1 was obtained as a yellow solid with a yield of 95%. 1 HNMR (300MHz, CDCl 3 )δ8.1...
Embodiment 2
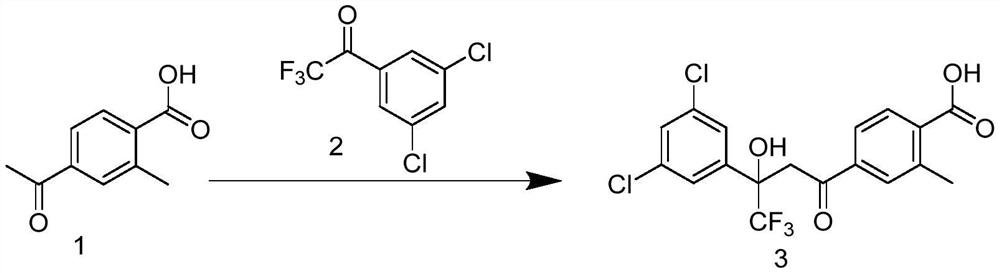
[0044] Example 2: Preparation of 4-(3-(3,5-dichlorophenyl)-4,4,4-trifluoro-3-hydroxybutyl)-benzoic acid (intermediate 3)
[0045]
[0046] Compound 1 (0.5g, 2.8mmol), compound 2 (0.68g, 2.8mmol), sodium lauricate (0.04g, 0.18mmol), potassium carbonate (0.6g, 4.34mmol), water (12mL, 0.66mol) Mix well, and stir at 60°C for 24 hours. At this time, the reactant becomes slightly white mud. Add water to the reaction liquid, adjust the pH to 1-2 with concentrated hydrochloric acid, extract with ethyl acetate, dry with anhydrous sodium sulfate, and purify by column chromatography. , the mobile phase was petroleum ether (PE) and ethyl acetate (EA), (PE:ethyl acetate EA=2:1, v:v), and 0.915g of a yellowish solid was obtained with a yield of 77.42%.
[0047] 1 HNMR (300MHz, CDCl 3 )δ8.21(d, J=8.3Hz, 1H), 7.87(d, J=7.5Hz, 2H), 7.55(d, J=1.8Hz, 2H), 7.41(d, J=1.8Hz, 1H) ,5.64(s,1H),3.92(d,J=17.6Hz,1H),3.76(d,J=17.6Hz,1H),2.78(s,3H).
Embodiment 3
[0048] Example 3: (1) Preparation of 4-(3-(3,5-dichlorophenyl)-4,4,4-trifluoro-2-enol)-benzoic acid (intermediate 4)
[0049]
[0050] Compound 3 (0.5g, 1.19mmol) was dissolved in 20mL of dichloromethane, and triethylamine (0.62g, 6.12mmol) was added under stirring to react at room temperature for 1h, and then the reaction solution was spin-dried to obtain triethylamine salt. Add 20mL of toluene and 4-dimethylaminopyridine (0.03g, 0.24mmol) to the ethylamine salt and heat to 60°C, add acetic anhydride (0.4mL, 4.2mmol) dropwise, after the addition is complete, heat up to 80°C , stirred for 6 hours, monitored for complete reaction, cooled to room temperature, added water, adjusted the pH to 1-2 with concentrated hydrochloric acid, extracted with EA, washed with water, washed with salt, dried over anhydrous sodium sulfate, and obtained 0.405 g of a yellow solid. The yield was 84.6%, and the next step was prepared.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com