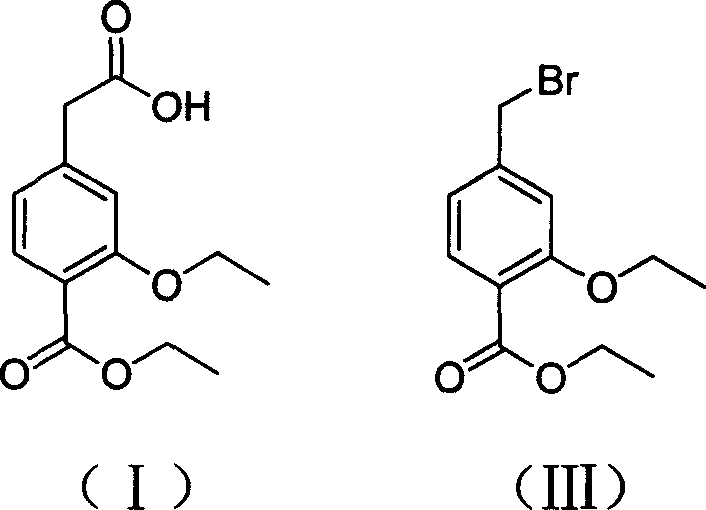
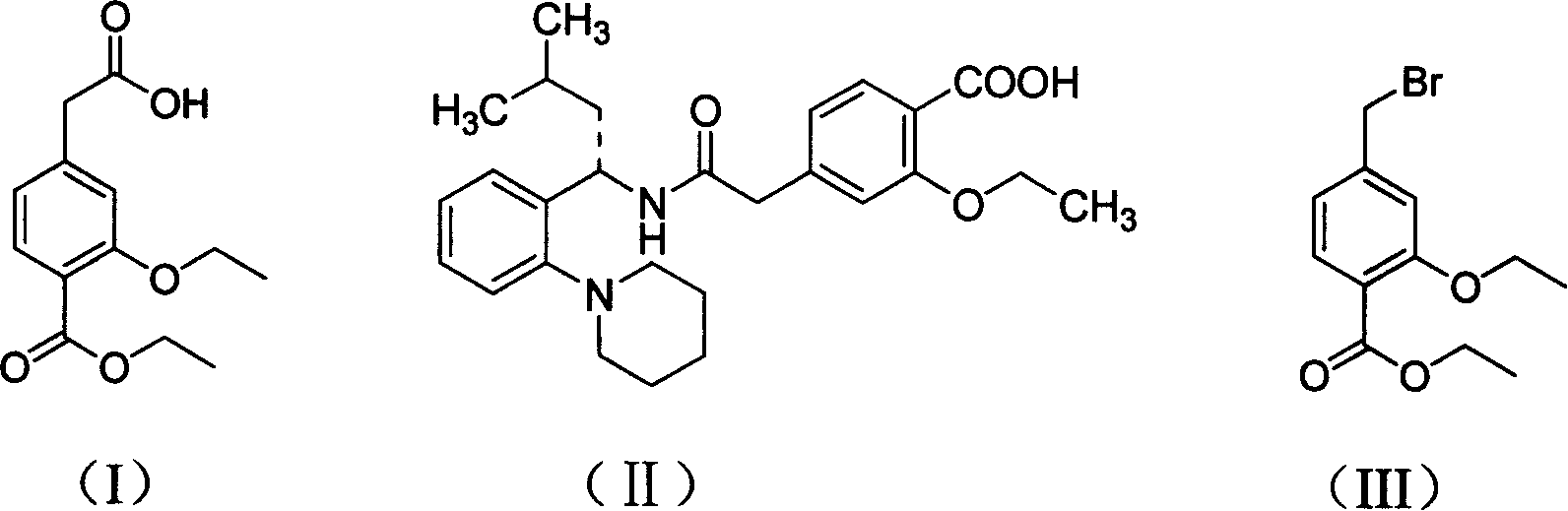
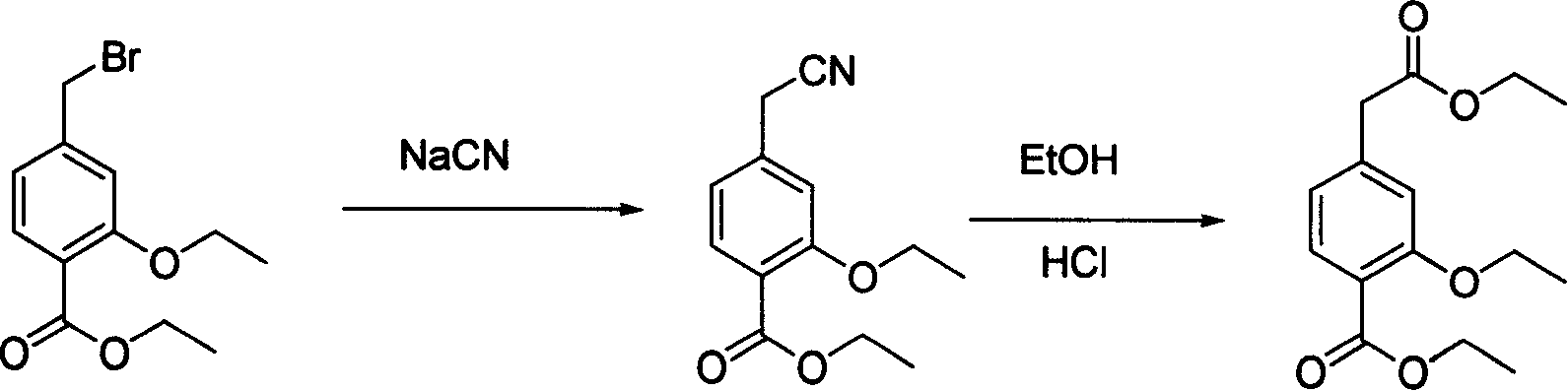
Novel method of producing repaglinide key intermediate
A technology of Grignard reagents and compounds, applied in the field of new preparation of 4-ethoxycarbonyl-3-ethoxyphenylacetic acid, can solve the problems of many side reactions, difficult purification of products, cumbersome operation, etc., and increase the safety of the reaction , shorten the reaction steps, reduce the effect of by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] N 2 Under protection, add 15g of magnesium chips into the dry reaction flask, add 30ml of tetrahydrofuran to cover the magnesium chips, and slowly raise the temperature to initiate the Grignard reaction. Another 270ml of tetrahydrofuran was mixed with 28.6g of ethyl 4-bromomethyl-2-ethoxybenzoate (compound of formula (III)), and transferred into a constant pressure dropping funnel. Add dropwise under weak reflux, and continue the reflux reaction for 1 hour after the drop is completed. After cooling down to 0°C, the solution was poured into 8.8 g of dry ice. Add 100ml of water dropwise and stir for 1 hour. Acidify with 10% hydrochloric acid to adjust the pH value of the system to 2.0. Extract with toluene (60ml×2), dry over anhydrous sodium sulfate, and concentrate under reduced pressure to remove toluene. The residue was dropped into 150ml of n-hexane and stirred overnight at room temperature. Filter and dry to obtain 10 g of white solid with a yield of 40%.
Embodiment 2
[0021] N 2 Under protection, add 15g of magnesium chips into the dry reaction flask, take 30ml of isopropyl ether to cover the magnesium chips, and add a small grain of iodine to trigger the Grignard reaction. Another 270ml of isopropyl ether was mixed with 28.6g of ethyl 4-bromomethyl-2-ethoxybenzoate (compound of formula (III)), transferred into a constant pressure dropping funnel, and added dropwise under weak reflux. After dropping, react at room temperature for 1 hour. Cool down to -5°C, pour the solution into 8.8 g of dry ice, add 100 ml of water dropwise, and stir for 1 hour. Acidify with 10% hydrochloric acid to adjust the pH value of the system to 2.0. Extract with toluene (60ml×2), dry over anhydrous sodium sulfate, and concentrate under reduced pressure to remove toluene. The residue was dropped into 150ml of n-hexane and stirred overnight at room temperature. After filtering and drying, 9.8 g of white solid was obtained with a yield of 39%.
Embodiment 3
[0023] N 2 Under protection, add 15g of magnesium chips into a dry reaction flask, take 30ml of a mixture of isopropyl ether and tetrahydrofuran (the volume ratio of the two is 1:1) to cover the magnesium chips, and slowly raise the temperature to initiate a Grignard reaction. Take another 270ml isopropyl ether and tetrahydrofuran mixed solution (the volume ratio of the two is 1:1) and mix with 28.6g ethyl 4-bromomethyl-2-ethoxybenzoate (compound of formula (III)), transfer to constant pressure In the dropping funnel, add dropwise under weak reflux. After dropping, the reaction was carried out under reflux for 1 hour. Cool down to 5°C. 4.4 g of dry ice was added to the solution, 100 ml of water was added dropwise, and stirred for 1 hour. Acidify with 10% hydrochloric acid, adjust the pH of the system to 2.0, extract with toluene (60ml×2), dry over anhydrous sodium sulfate, and concentrate under reduced pressure to remove toluene. The residue was dropped into 150ml of n-hex...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com