Carboxyl-terminated polyether ionic liquid catalyst and preparation method thereof
An ionic liquid and carboxyl-terminated technology, which is applied in the field of carboxyl-terminated polyether ionic liquid catalyst and its preparation, can solve the problems of insufficient catalyst efficiency and selectivity, short cycle life, harsh reaction conditions, etc., and achieve synergistic catalytic effect , long cycle life, reduce the effect of by-product formation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
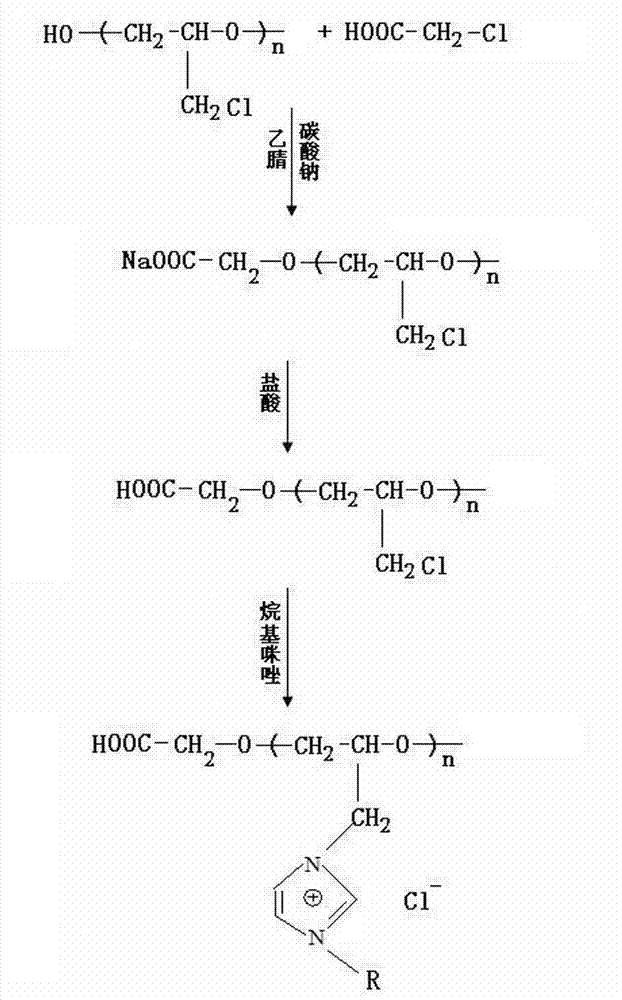
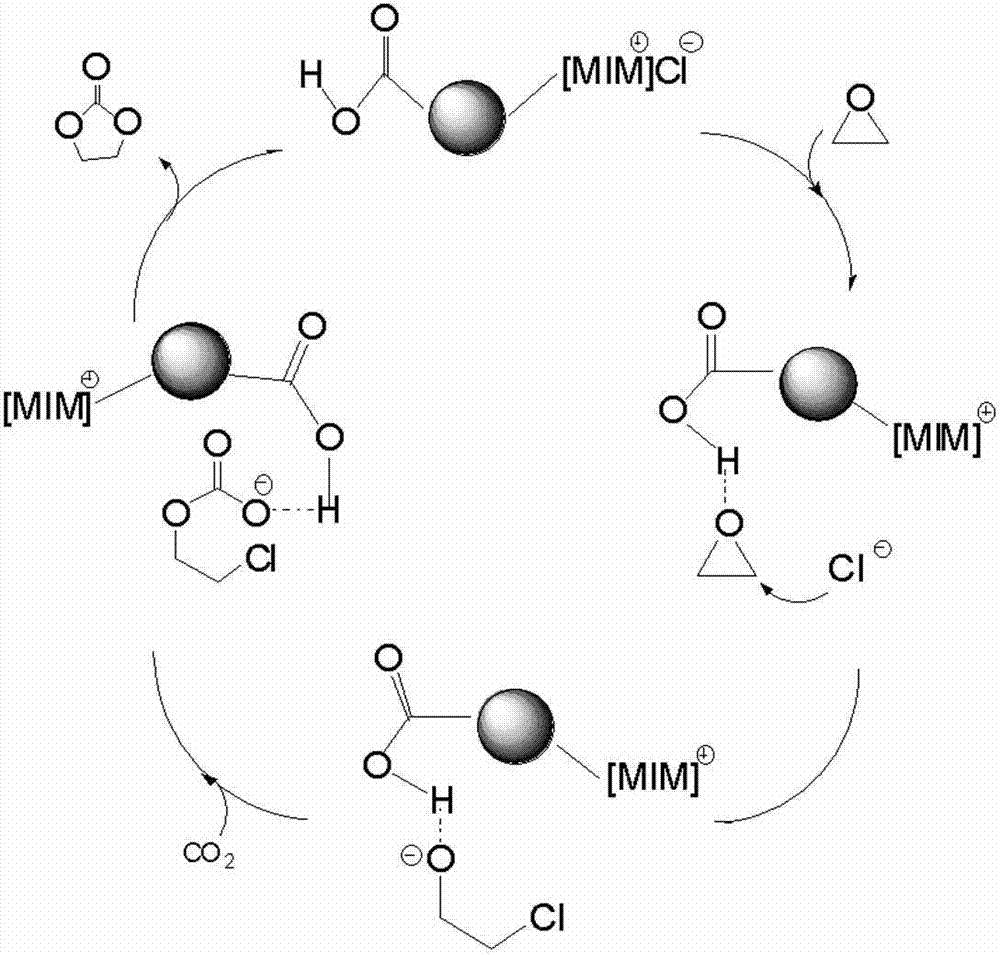
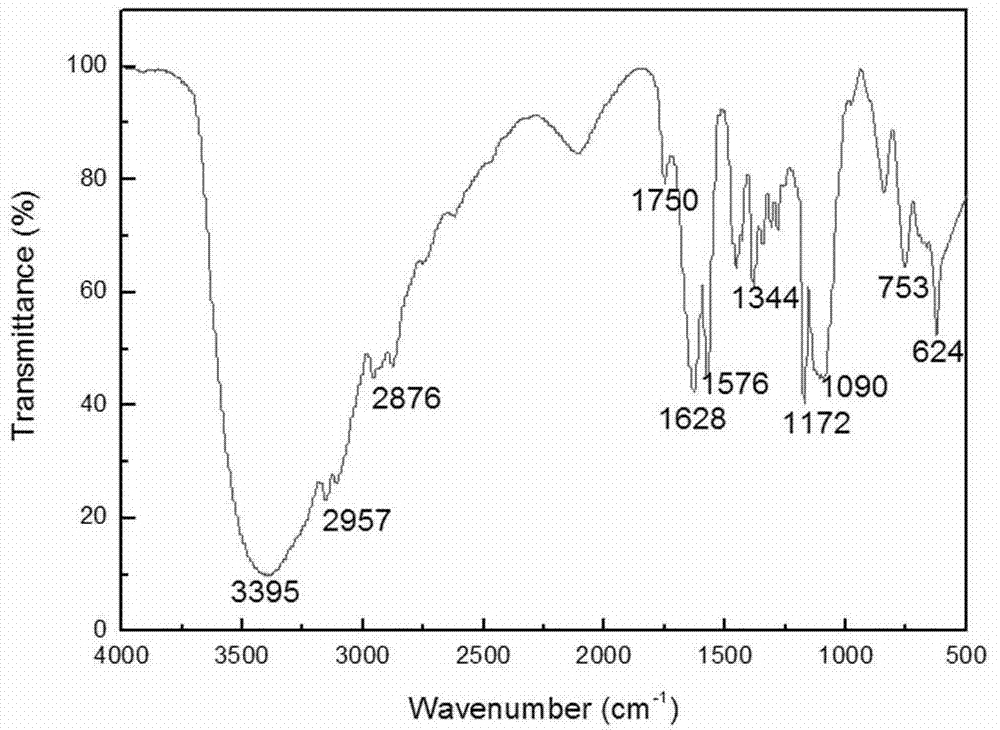
[0059] First, add hydroxyl-terminated polyepichlorohydrin to chloroacetic acid in a molar ratio of 1:2.2 into a three-necked flask equipped with a condenser, acetonitrile as a solvent, and start stirring at a temperature of 80°C. After reacting for 1 hour, a small amount of Na 2 CO 3 Add the powder into the three-necked flask until it is completely dissolved, then repeat the addition until the system can no longer be dissolved. After reacting for about 7-8 hours, hydrochloric acid was added dropwise to the obtained liquid to adjust the pH value to be acidic. During the dropping process, the system was layered, the upper layer was a liquid phase, and the lower layer was a granular white solid. The upper liquid phase was rotary evaporated at a temperature of 70° C. and 0.09 MPa to remove the acetonitrile solvent. Then the product was washed 5 times with deionized water to remove some small molecules and excess chloroacetic acid, and then rotated at 70°C and 0.09Mpa to remove a ...
Embodiment 2
[0063]In the same equipment used in Example 1, under the same conditions, just change the catalyst consumption to 5.40g (accounting for 5% of the propylene oxide quality), its catalyzed reaction nearly 90min observes that the carbon dioxide mass flow count value does not change, After the reaction was completed, the purity of the propylene carbonate product was measured to be 99.6%, the calculated conversion rate of propylene oxide was 100.0%, and the selectivity was 99.2%.
Embodiment 3
[0065] In the same equipment used in Example 1, under the same conditions, just change the catalyst consumption to 1.08g (accounting for 1% of the propylene oxide mass), react 2.5h and observe that the carbon dioxide mass flow count value does not change, and the reaction is completed The purity of the propylene carbonate product was measured to be 99.1%, the conversion rate of ethylene oxide was calculated to be 99.8%, and the selectivity of ethylene carbonate was 98.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com