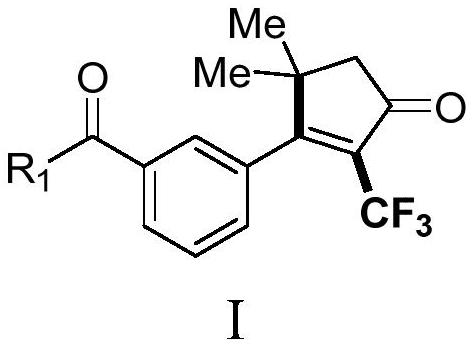
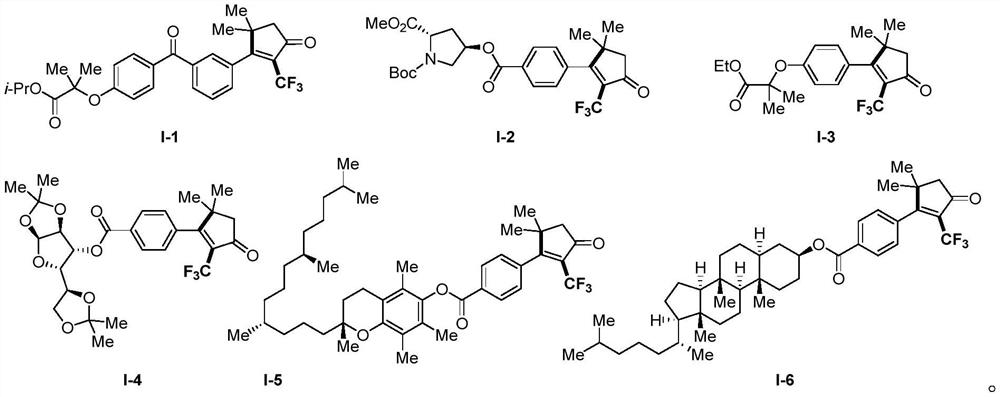
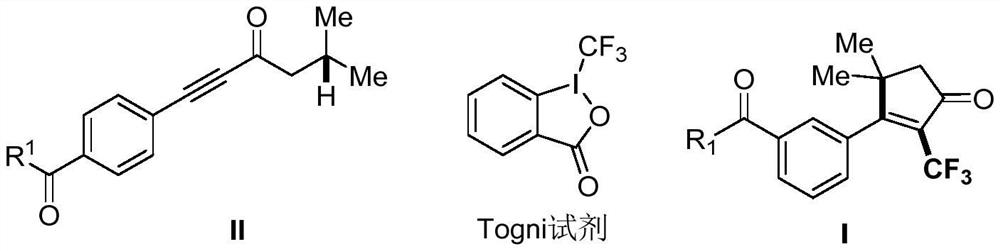
Derivative containing 2-trifluoromethyl cyclopentenone and preparation method of derivative
A technology for trifluoromethyl cyclopentenone and derivatives, which is applied in the field of derivatives containing 2-trifluoromethyl cyclopentenone and their preparation, can solve the problems of unsatisfactory free radical regioselectivity and the like, and achieves overcoming The effects of unsatisfactory regioselectivity, mild reaction conditions, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] CuCN (1.8mg, 0.02mmol), Togni reagent (94.8mg, 0.3mmol) and K 2 CO 3 (55.3mg, 0.4mmol), after vacuuming and changing nitrogen three times, add II-1 fenofibrate acetylene derivative (37.2mg, 0.2mmol) dissolved in 2mL of ethyl acetate acetonitrile. After the system was reacted at 50°C for 10 hours, water was added to the reaction system to quench the reaction, the reaction was extracted three times with ethyl acetate, the organic phases were combined, washed with saturated sodium chloride solution and dried with anhydrous sodium sulfate, and the solvent was spin-dried. Column chromatography was used to separate petroleum ethers / EtOAc=10:1 to obtain white solid I-1, yield: 56%; 1 H NMR (600MHz, CDCl 3 )δ7.83(d, J=8.2Hz, 2H), 7.79(d, J=8.8Hz, 2H), 7.27-7.23(m, 2H), 6.91-6.87(m, 2H), 5.13-5.06(m ,1H),2.62(s,2H),1.68(s,6H),1.29(s,6H),1.21(d,J=6.3Hz,6H). 13 C NMR (151MHz, CDCl 3 )δ200.07, 194.61, 173.07, 159.92, 138.55, 136.05, 132.09, 130.25 (q, J=31.2Hz), 130.01, 129.77...
Embodiment 2
[0040] Except that the amino acid acetylenone derivative shown in structural formula II-2 is used to replace the fenofibrate acetylenic ketone derivative shown in the structural formula in Example II-3, the rest of the operating steps are the same as in Example 1, and the white solid I is separated and obtained -2, yield: 80%, mp127-129°C, dr=1.3:1; petroleum ethers / EtOAc=5:1; 1 H NMR (600MHz, CDCl 3 )δ8.11(d, J=8.2Hz, 2H), 7.27-7.23(m, 2H), 5.60-5.56(m, 1H), 4.55(t, J=7.9Hz, 0.43H, minorrotamer), 4.45( t,J=8.0Hz,0.57H,major rotamer),3.87(d,J=3.1Hz,1H),3.80(s,1.29H,minor rotamer),3.79(s,1.71H,major rotamer),3.75- 3.73(m,1H),2.62(s,1H),2.60-2.53(m,2H),2.40-2.35(m,1H),1.48(s,3.90H,minor rotamer),1.46(s,5.10H, major rotamer), 1.28(s,6H). 13 C NMR (151MHz, CDCl 3 )δ199.87, 183.63, 173.01, 165.11, 153.60, 137.47, 130.29 (q, J=31.5Hz), 130.16, 129.44, 120.66 (q, J=273.4Hz), 80.73, 72.98, 57.99, 53.37, 52.8075, 5 ,36.70,28.24,26.60. 19 F NMR (565MHz, CDCl 3 )δ-60.38.HRMS(ESI)m...
Embodiment 3
[0044] Except that the fenofibrate acetylenone derivative shown in the structural formula I-1 in Example 1 is replaced by the clofibrate acetylenic ketone derivative shown in the structural formula II-3, the remaining operating steps are the same as in Example 1, and the separation is obtained Yellow liquid I-3, yield: 81%, petroleum ethers / EtOAc=10:1; 1 H NMR (600MHz, CDCl 3 )δ7.02(d, J=8.7Hz, 2H), 6.88(d, J=8.7Hz, 2H), 4.23(q, J=7.1Hz, 2H), 2.55(s, 2H), 1.64(s, 6H), 1.24(s, 6H), 1.21(t, J=7.1Hz, 3H). 13 C NMR (151MHz, CDCl 3 )δ200.58, 185.51, 173.91, 156.31, 129.77 (q, J=30.7Hz), 127.44, 125.69, 120.95 (q, J=273.4Hz), 118.02, 79.34, 61.55, 51.13, 43.07, 26.75, 235.974.1 19 F NMR (565MHz, CDCl 3 )δ-60.35.HRMS(ESI)m / z:[M+H] + Calcd for C 20 h 23 f 3 o 4 +H + :385.1621; Found 385.1626.
[0045] The reaction formula is as follows:
[0046]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com