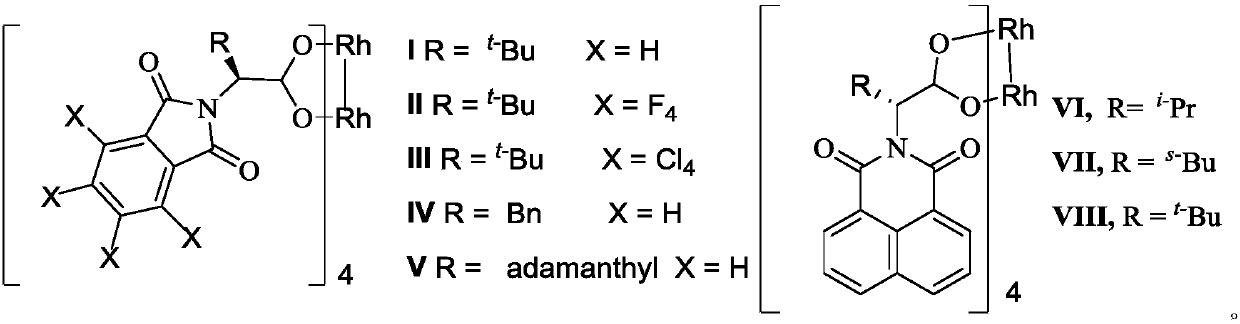Optically active indoline cyclopentenone and its derivatives and preparation method
A technology of indole spirocyclopentenone and optical activity, applied in organic chemistry methods, organic chemistry, etc., can solve the problem of rare cyclopentenone and achieve high yield and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
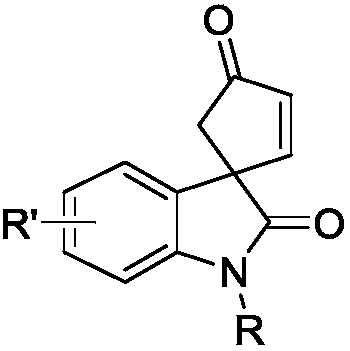
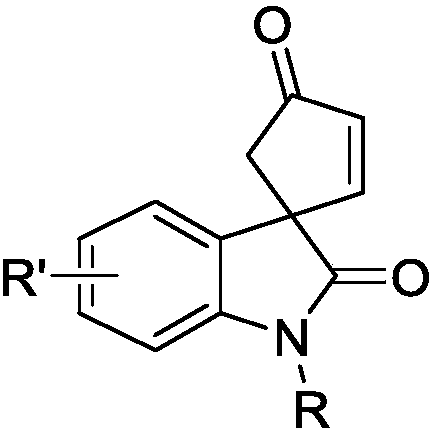
[0018] The synthesis of optically active indole spirocyclopentenone 3a, its reaction formula is:
[0019]
[0020] Add tetrakis[(S)-(-)-N-(p-dodecylbenzenesulfonyl)proline]dirhodium(II)(Rh 2 (DOSP) 4 (9.48mg, 0.005mmol), 2-trimethylsilyloxy-4-methoxy-1,3-butadiene (1.5mmol), add anhydrous and oxygen-free dichloromethane (1mL), stir at room temperature After 30min, a solution of N-benzyl-2-oxo-3-diazoindole (1a, 0.1mmol) dissolved in dichloromethane (1mL) was slowly added thereto, and after the reaction was completed (detecting the reaction by TLC progress), the reaction mixture was lowered to 0°C, added 0.5mL trifluoroacetic acid and stirred for 15min, the reaction mixture was added 5mL saturated NH 4 Cl aqueous solution, the reaction solution was extracted three times with ethyl acetate, 10 mL each time, the organic phases were combined and dried over anhydrous sodium sulfate. After evaporating the solvent, separation and purification by column chromatography (petroleum...
Embodiment 2
[0022] The synthesis of optically active indole spirocyclopentenone 3a, its reaction formula is:
[0023]
[0024] Add chiral rhodium carboxylate I (1.42mg, 0.001mmol), 2-trimethylsilyloxy-4-methoxy-1,3-butadiene (2mmol) into a dry test tube Oxygen carbon tetrachloride (1mL), after stirring at room temperature for 30min, slowly added N-benzyl-2-oxo-3-diazoindole (1a, 0.1mmol) dissolved in carbon tetrachloride ( 1mL) solution, after the reaction was completed (reaction progress was detected by TLC), the reaction compound was lowered to 0°C, 0.5mL trifluoroacetic acid was added and stirred for 15min, and the reaction mixture was added 5mL saturated NH 4 Cl aqueous solution, the reaction solution was extracted three times with ethyl acetate, 10 mL each time, the organic phases were combined and dried over anhydrous sodium sulfate. After evaporating the solvent, separation and purification by column chromatography (petroleum ether: ethyl acetate = 10:1) gave (S)-2-oxo-indole-3...
Embodiment 3
[0027] The synthesis of optically active indole spirocyclopentenone 3a, its reaction formula is:
[0028]
[0029] Add chiral rhodium carboxylate II (2.67mg, 0.002mmol), 2-triethylsilyloxy-4-methoxy-1,3-butadiene (2mmol) into a dry test tube, add anhydrous Oxygen-free carbon tetrachloride (1mL), after stirring at 0°C for 30min, slowly added N-benzyl-2-oxo-3-diazoindole (1a, 0.1mmol) dissolved in tetrachloride Carbon dioxide (1 mL) solution, after the completion of the reaction (reaction progress detected by TLC), the reaction compound was lowered to 0 ° C, 0.5 mL of trifluoroacetic acid was added and stirred for 15 min, and the reaction mixture was added with 5 mL of saturated NH 4 Cl aqueous solution, the reaction solution was extracted three times with ethyl acetate, 10 mL each time, the organic phases were combined and dried over anhydrous sodium sulfate. After evaporating the solvent, separation and purification by column chromatography (petroleum ether: ethyl acetate ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com