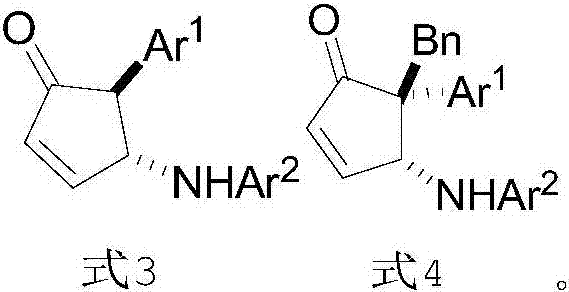
Preparation method and application of chiral 4-amino-cyclopentenone
A technology of aminocyclopentenone and chirality, which is applied in the field of asymmetric catalytic synthesis, can solve the problems of limiting large-scale synthesis and application, cumbersome operation, and lengthy steps, and achieves high selectivity, high purity, and simple reaction conditions easy to control effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
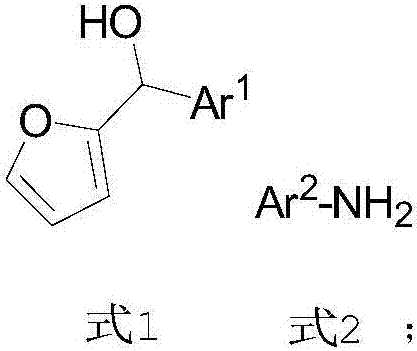
[0033] The embodiment of the present invention provides a preparation method of chiral 4-aminocyclopentenone, comprising the following steps:
[0034] S01. provide 2-furyl alcohol as shown in formula 1, aromatic amine and catalyst CPA shown in formula 2, wherein, said CPA is the phosphoric acid of chiral spiro ring skeleton substituted by 1-pyrenyl,
[0035]
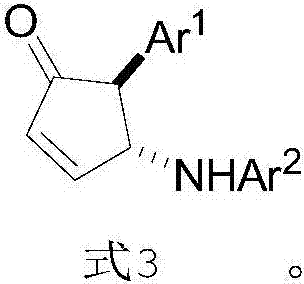
[0036] S02. After dissolving the aromatic amine and the CPA in the first organic solvent, cool to 0-5°C, then add the solution of the 2-furyl alcohol in the first organic solvent, and perform asymmetric Intermolecular aza Piancatelli rearrangement reaction, to obtain chiral 4-aminocyclopentenone shown in formula 3,
[0037]
[0038] Specifically, in the above step S01, in the formula 1 structure of the 2-furyl alcohol, the Ar 1 It is one of a benzene ring or a substituted benzene ring, a fused ring or a substituted fused ring, and a heterocyclic ring. More specifically, in the substituted benzene ring and the sub...
Embodiment 1
[0076] Using Ar 1 2-furyl alcohol and Ar as Ph 2 is 3,5-(CF 3 ) 2 C 6 h 3 The arylamine is used as a reaction raw material, and is reacted with a chiral phosphoric acid catalyst, and the specific implementation process is as follows:
[0077] Dissolve 3,5-bistrifluoromethylaniline (172mg, 0.75mmol) and catalyst (35.7mg, 0.05mmol) in 1,2-dichloroethane (10mL), cool to 0°C after 30 minutes, slowly A solution of phenyl-substituted 2-furyl alcohol (87.0 mg, 0.5 mmol) in 1,2-dichloroethane (10 mL) was added dropwise; after that, it was stirred at room temperature for 75 hours. Its reaction equation is as follows:
[0078]
[0079] The reaction solution was directly applied to silica gel column chromatography to obtain 126 mg of brown oily product, and the calculated yield was 66%.
[0080] After the preparation of this step is completed, in order to further verify that the purified compound is indeed the target product to be prepared in this example, the obtained oily pro...
Embodiment 2
[0089] Using Ar 1 = Ph of 2-furyl alcohol and Ar 2 =2,5-(CF 3 ) 2 C 6 h 3 The arylamine was used as the reaction raw material, and was reacted with 10% chiral phosphoric acid catalyst. According to the same steps described in the above example 1, the reaction was carried out for 22 hours to obtain 95 mg of yellow oily liquid product, and the calculated yield was 83%.
[0090] Similarly, after the preparation in this step is completed, the obtained oily product is analyzed by means of measuring the specific rotation, high performance liquid chromatography to measure the ee value, nuclear magnetic resonance, infrared and high-resolution mass spectrometry. Among them, the test analysis data are as follows:
[0091] 1. Specific rotation [α] measured by D-line at 23°CD 23 : -37.9 (c=1.4, CHCl 3 ).
[0092] 2. Determination of ee value by high performance liquid chromatography: chiral column Daicel IC column; 3% i-PrOHinhexanes; 1.0mL / min; retention time: 17.2min (major), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com