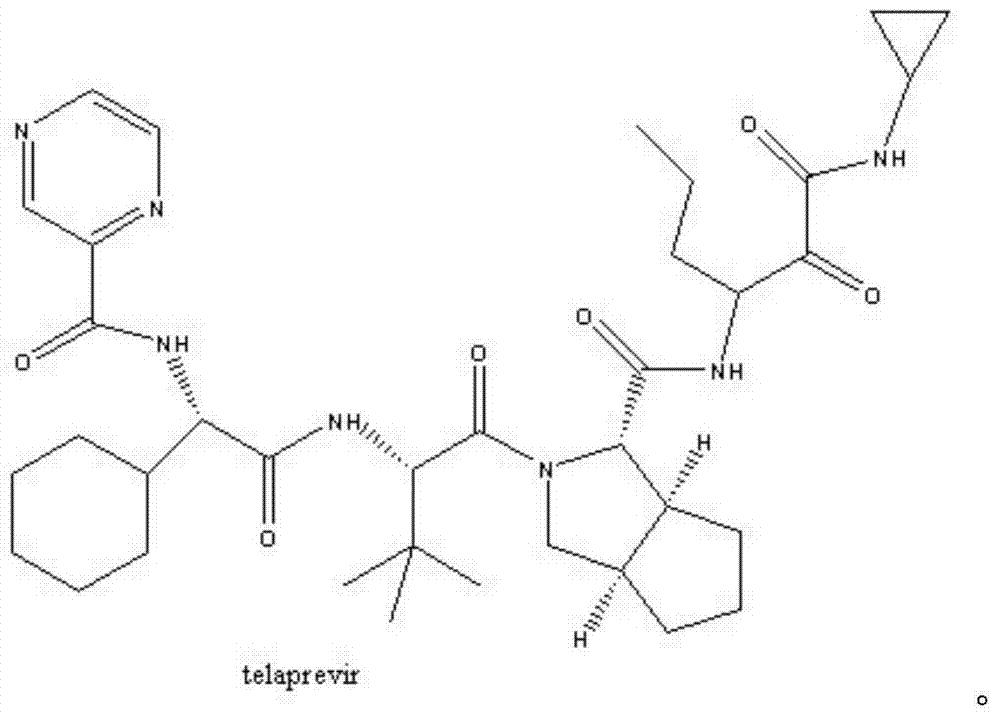
Synthetic method of telaprevir intermediate
A synthetic method and technology of telaprevir are applied in the field of drug synthesis, and can solve problems such as unfavorable large-scale industrial production, potential safety hazards, long reaction steps, etc., to avoid chiral racemization phenomenon, suitable for large-scale industrial production, Simple to use effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
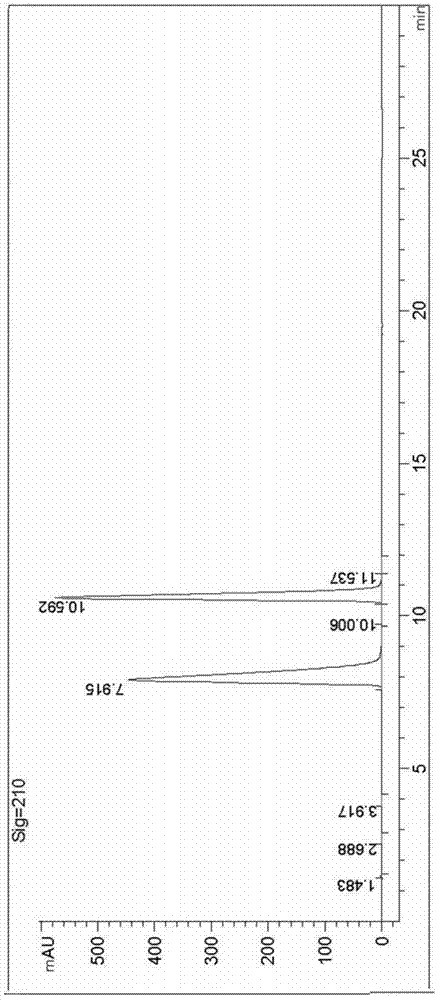
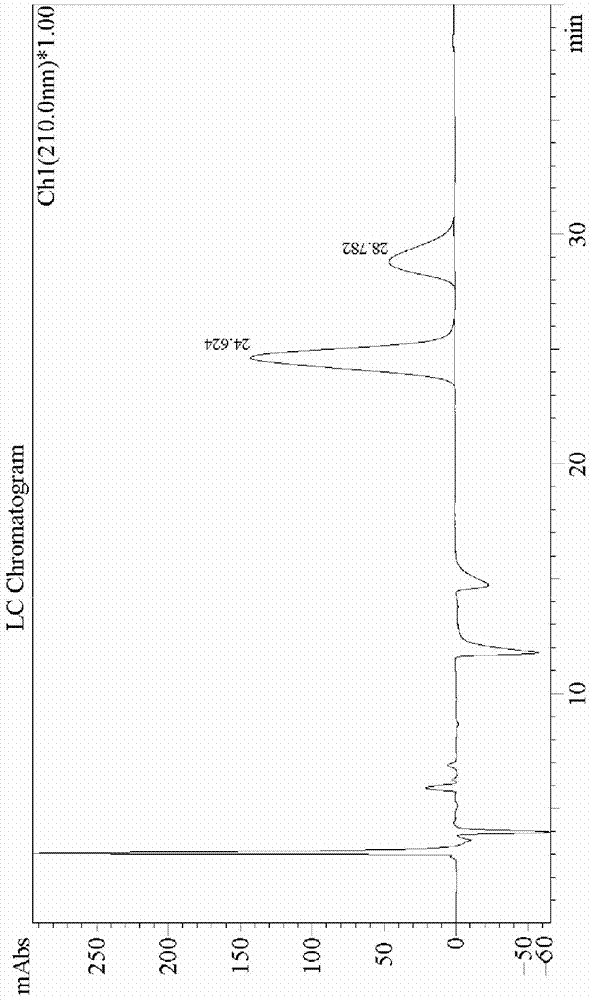
Image
Examples
Embodiment 1
[0039]Add 100mL of formic acid to the three-necked reaction flask, add 10.5g of sodium percarbonate, 20g of ethyl butyryl acetate, and 1.3g of iodobenzene in sequence at 15-20°C. After the addition, continue to keep the internal temperature at 15-20°C for 2 hours. The complete conversion of the raw material was monitored by gas chromatography, the solvent formic acid was removed by concentration, and the residue was distilled under reduced pressure to obtain 19 g of intermediate B with a yield of 86.3%.
[0040] Make 13.75g of ammonium formate and 19g of intermediate B into a 100mL solution, adjust the pH of the solution to 8 with ammonia water, then add 0.3g of nicotinamide adenine dinucleotide (NAD) and 0.07g of dithiothreitol to obtain a mixed solution , adding the mixed solution into a 100mL solution containing 0.5g Pichia pastoris extract to form a reaction solution, the cell extract contains phenylalanine dehydrogenase and formate dehydrogenase by measuring; the reaction ...
Embodiment 2
[0043] Add 200mL of formic acid to the three-necked reaction flask, add 10.3g of sodium perborate, 39g of ethyl butyroacetate, and 2.2g of iodobenzene at 15-20°C. The complete conversion of raw materials was monitored by chromatography, the solvent formic acid was removed by concentration, and the residue was distilled under reduced pressure to obtain 27.3 g of intermediate B with a yield of 63.5%.
[0044] Make 19.8g of ammonium formate and 27.3g of intermediate B into a 150mL solution, adjust the pH of the solution to 8 with ammonia water, then add 0.44g of nicotinamide adenine dinucleotide (NAD) and 0.1g of dithiothreitol to obtain a mixture solution, the mixed solution was added into a 150mL solution containing 1.0g Pichia pastoris extract to form a reaction solution, and the cell extract contained phenylalanine dehydrogenase and formate dehydrogenase by measuring; Keep the temperature of the reaction solution at 35-40°C, stir at a speed of 80rpm, maintain the pH of the re...
Embodiment 3
[0047] Add 15mL of acetonitrile, 15mL of hydrogen peroxide, and 7g of sodium carbonate to the three-necked reaction flask in sequence. After stirring for 0.5 hours, continue to add 100mL of formic acid, 20g of ethyl butyryl acetate, and 1.3g of iodobenzene. After 2.5 hours, the complete conversion of the raw material was monitored by gas chromatography, the solvent formic acid was removed by concentration, and the residue was distilled under reduced pressure to obtain 8.5 g of intermediate B with a yield of 38.6%.
[0048] Prepare 6.15g of ammonium formate and 8.5g of intermediate B into a 50mL solution, adjust the pH of the solution to 8 with ammonia water, then add 0.13g of nicotinamide adenine dinucleotide (NAD) and 0.04g of dithiothreitol to obtain a mixture solution, the mixed solution was added into a 50mL solution containing 0.25g Pichia pastoris extract to form a reaction solution, and the cell extract contained phenylalanine dehydrogenase and formate dehydrogenase by m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com