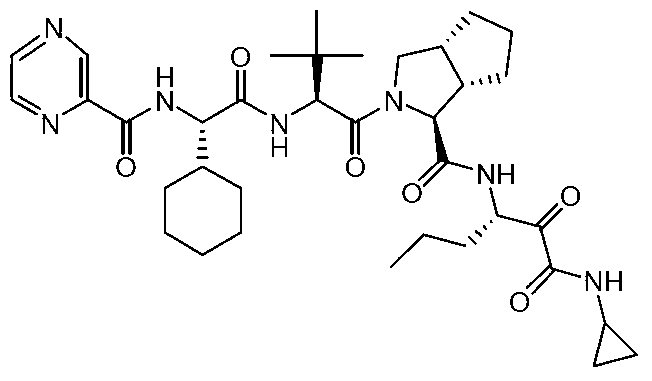
Synthesis method of telaprevir
A synthetic method, the technology of telaprevir, applied in the field of drug synthesis, can solve the problems of high reagent cost and post-processing cost, cumbersome post-processing steps, and high cost, and achieve the goal of reducing yield and purity, reducing yield and purity Effect of loss, short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
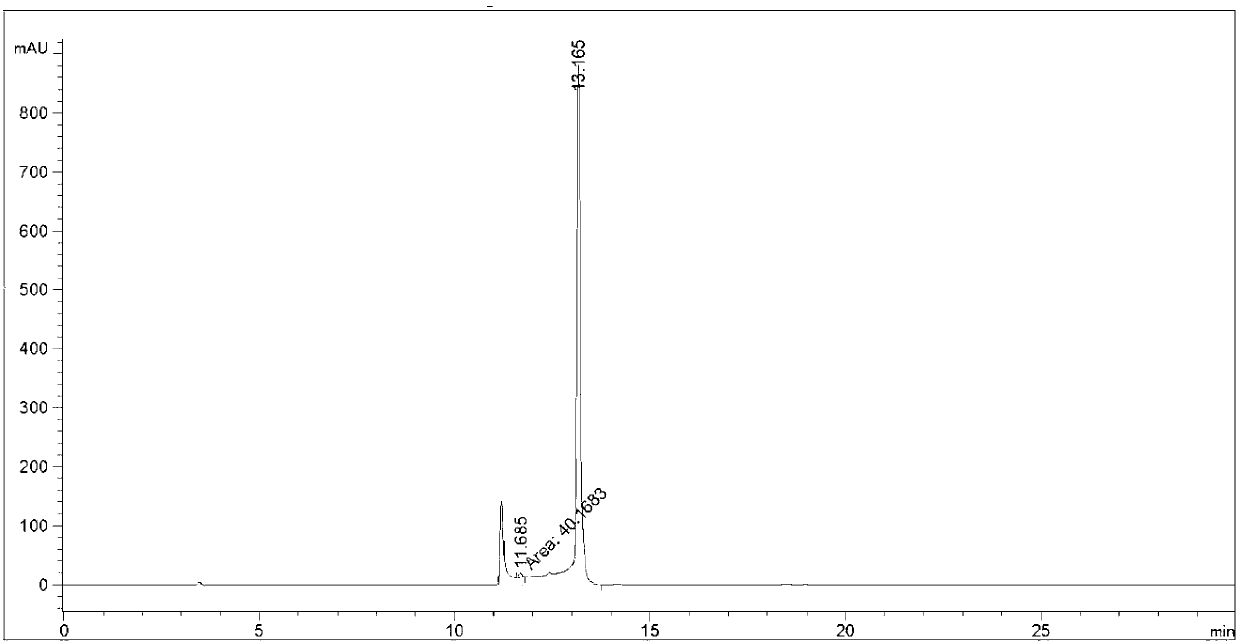
[0042] At -10°C, 25g (2S)-2-cyclohexyl-N-(2-pyrazinylcarbonyl)glycyl-3-methyl-L-valine, 10g (1S,3aR,6aS)- Add tert-butyl octahydrocyclopenta[c]pyrrole-1-carboxylate, 13g N,N'-diisopropylcarbodiimide into a reaction flask of 40mL dichloromethane, keep stirring at -10°C for 10 minutes , the mixture was slowly warmed to room temperature and stirred overnight at room temperature. After the reaction was detected by TLC and HPLC, the temperature of the reaction solution was lowered to 3° C., stirred for 10 minutes, and then suction-filtered to obtain a white filter cake and a yellow-green filtrate. The filter cake was washed twice with 20 mL of dichloromethane. Drained, combined filtrate, washed three times with 100mL of water, 100mL of 1N hydrochloric acid once, 100mL of saturated sodium bicarbonate aqueous solution once, then washed once with 100mL of saturated brine, dried with 10g of anhydrous sodium sulfate and concentrated to obtain the intermediate product (1 ).
[0043] A...
Embodiment 2
[0046] At -15°C, 50g (2S)-2-cyclohexyl-N-(2-pyrazinylcarbonyl)glycyl-3-methyl-L-valine, 20g (1S,3aR,6aS)- Add tert-butyl octahydrocyclopenta[c]pyrrole-1-carboxylate, 32g N,N-dicarbonylimidazole into a reaction flask of 80mL N,N-dimethylformamide, keep stirring at -15°C for 15 minutes , the mixture was slowly warmed to room temperature and stirred overnight at room temperature. After the reaction was detected by TLC and HPLC, the temperature of the reaction solution was lowered to 5° C., stirred for 15 minutes, and then suction-filtered to obtain a white filter cake and a yellow-green filtrate. The filter cake was washed twice with 40 mL of dichloromethane. Drained, combined filtrate, washed three times with 200mL water, 200mL1N hydrochloric acid washed once, 200mL saturated sodium bicarbonate aqueous solution washed once, then washed once with 200mL saturated brine, 20g of anhydrous sodium sulfate dried and concentrated to obtain the intermediate product (1 ).
[0047] Afte...
Embodiment 3
[0050]At -5°C, 12.5g (2S)-2-cyclohexyl-N-(2-pyrazinylcarbonyl) glycyl-3-methyl-L-valine, 5g (1S, 3aR, 6aS) - tert-butyl octahydrocyclopenta[c]pyrrole-1-carboxylate, 8.0 g of 3,4-dihydro-3-hydroxy-4-oxo-1,2,3-benzotriazine added to 20 mL In a reaction flask of dimethyl sulfoxide, the mixture was stirred at -5°C for 5 minutes, and then the mixture was slowly warmed to room temperature and stirred overnight at room temperature. After the reaction was detected by TLC and HPLC, the temperature of the reaction solution was lowered to -2°C, stirred for 6 minutes, and suction filtered to obtain a white filter cake and a yellow-green filtrate. The filter cake was washed twice with 10 mL of dichloromethane. Drained, combined filtrate, washed three times with 50mL of water, washed once with 50mL of 1N hydrochloric acid, washed once with saturated aqueous sodium bicarbonate solution of 50mL, washed once with saturated brine of 50mL, dried with 5g of anhydrous sodium sulfate and concentra...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com