Synthesis method of Telaprevir intermediate
A technology of telaprevir and synthetic method, which is applied in the field of drug synthesis, can solve problems such as high energy consumption and equipment occupancy rate, health hazards of operators, and easy pollution of the environment, so as to improve conversion rate and yield and shorten reaction time , reduce the effect of side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
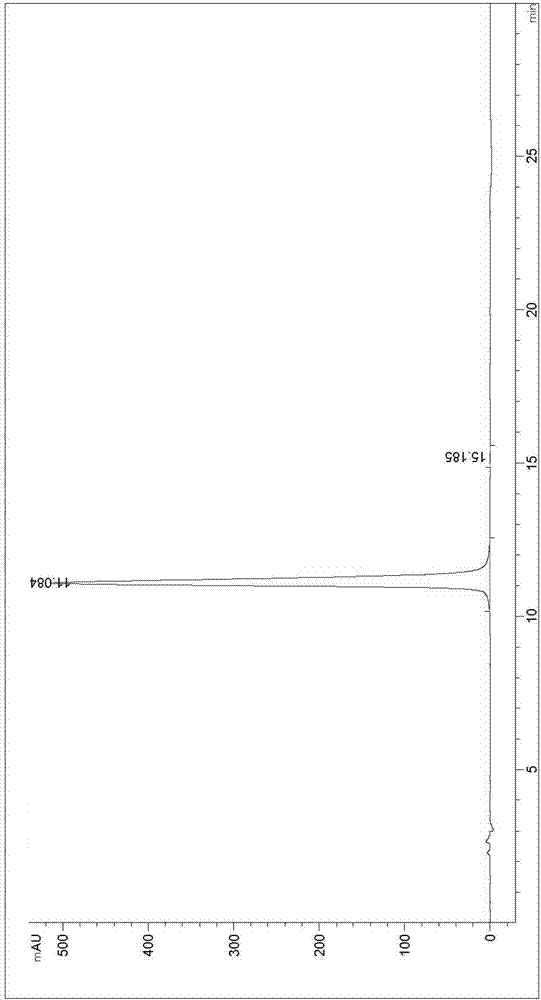
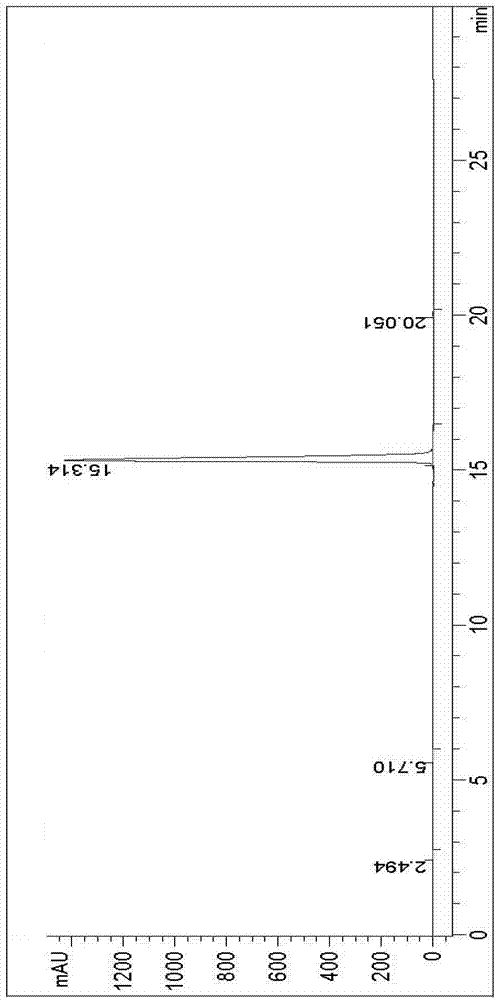
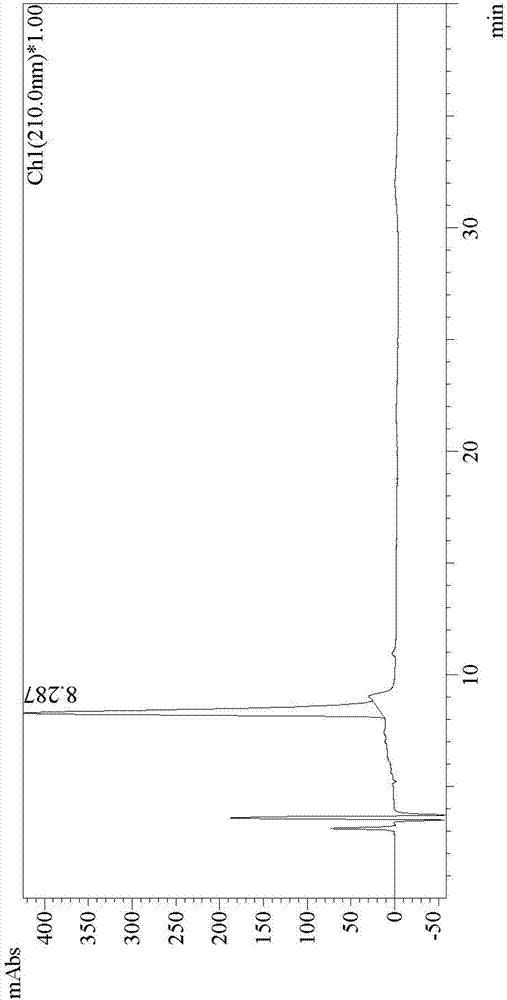
Image
Examples
Embodiment 1
[0056] At room temperature, 1.58g of ethyl 2-oxohexanoate and 1.82g of 1,8-diazabicyclo[5.4.0]undec-7-ene were dissolved in 20mL of acetonitrile to form a reaction solution. The diimide was added to the reaction solution, and the mixture was stirred at room temperature for 12 hours until the reaction was completed as detected by TLC. The reaction solution after the completion of the reaction was poured into 100 mL of water, followed by extraction with dichloromethane three times, and the organic phases were combined. The organic phase was washed three times with 100 mL of water, dried with anhydrous magnesium sulfate, suction filtered to obtain a filtrate, the filtrate was concentrated under reduced pressure, and recrystallized with n-heptane and ethyl acetate to obtain 2.22 g of (S)-3-(2,5-dioxane) pyrrol-1-yl)-2-oxohexanoic acid ethyl ester, the yield is 87%, and the purity is 99.50%.
[0057]2.22g of (S)-3-(2,5-dioxopyrrol-1-yl)-2-oxohexanoic acid ethyl ester prepared abov...
Embodiment 2
[0063] At room temperature, 3.16g of ethyl 2-oxohexanoate and 3.64g of 1,8-diazabicyclo[5.4.0]undec-7-ene were dissolved in 40mL of tetrahydrofuran to form a reaction solution, and 4.28g of N-bromobutan The diimide was added to the reaction solution, and the mixture was stirred at room temperature for 14 hours until the reaction was completed as detected by TLC. The reaction solution after the completion of the reaction was poured into 200 mL of water, extracted three times with dichloromethane, and the organic phases were combined. The organic phase was washed three times with 200 mL of water, dried with anhydrous magnesium sulfate, suction filtered to obtain a filtrate, the filtrate was concentrated under reduced pressure, and recrystallized with n-heptane and ethyl acetate to obtain 4.44 g of (S)-3-(2,5-dioxane) pyrrol-1-yl)-2-oxohexanoic acid ethyl ester.
[0064] 4.44g (S)-3-(2,5-dioxopyrrol-1-yl)-2-oxohexanoic acid ethyl ester prepared above was added to a 250mL three-n...
Embodiment 3
[0070] At room temperature, 4.74g of ethyl 2-oxohexanoate and 5.46g of 1,8-diazabicyclo[5.4.0]undec-7-ene were dissolved in 60mL of dichloromethane to form a reaction solution, and 6.42g of N-bromo The succinimide was added to the reaction solution, and the mixture was stirred at room temperature for 15 hours until the reaction was completed as detected by TLC. The reaction solution after the completion of the reaction was poured into 300 mL of water, followed by extraction with dichloromethane three times, and the organic phases were combined. The organic phase was washed three times with 300 mL of water, dried with anhydrous magnesium sulfate, suction filtered to obtain a filtrate, the filtrate was concentrated under reduced pressure, and recrystallized with n-heptane and ethyl acetate to obtain 6.66 g of (S)-3-(2,5-dioxane) pyrrol-1-yl)-2-oxohexanoic acid ethyl ester.
[0071] The 6.66g (S)-3-(2,5-dioxopyrrol-1-yl)-2-oxohexanoic acid ethyl ester obtained above was added to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com