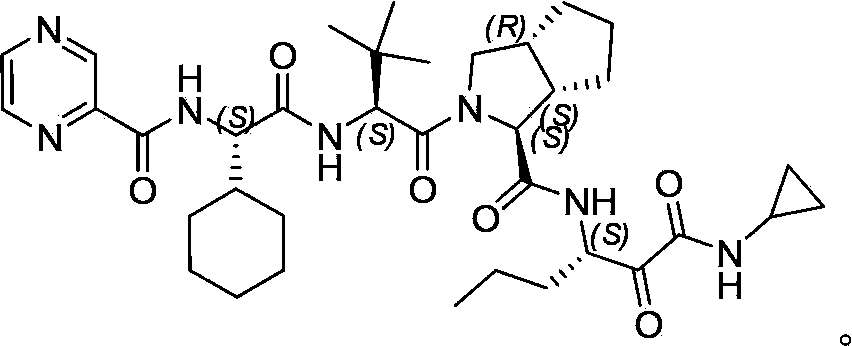
Method for preparing telaprevir intermediate
A technology of vir intermediates and telapid, which is applied in the field of chemical drug synthesis, can solve problems such as not suitable for industrial production, and achieve the effect of simplifying operations and improving yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
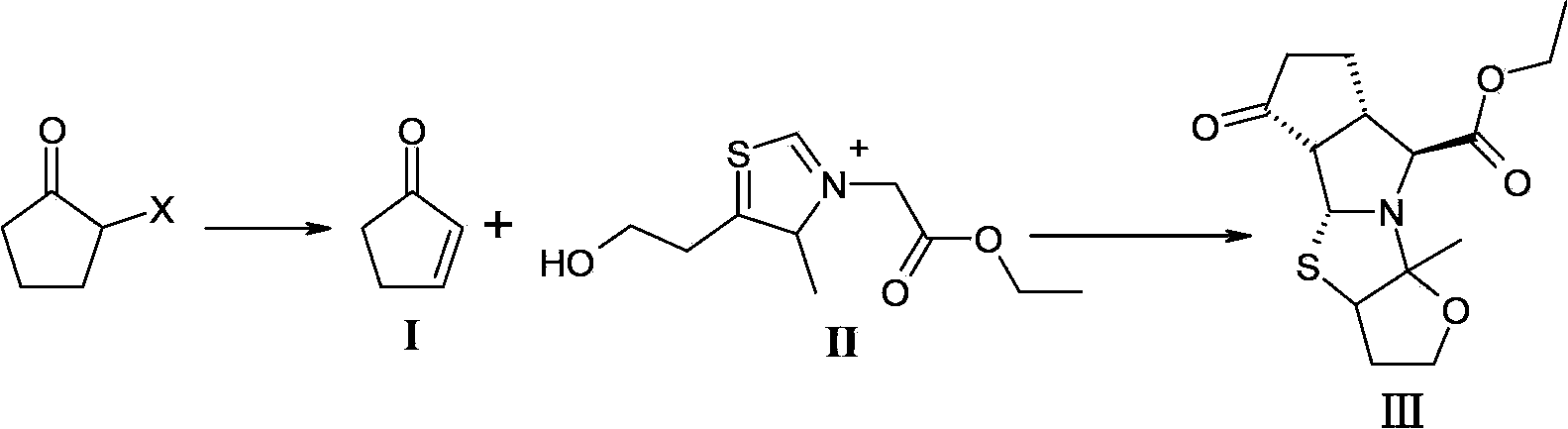
[0025] Add 300mL DMF, 27.0g (0.6mol) LiCO 3 , 2.1g (0.04mol) LiBr, 0.1g (0.0015mol) hydroquinone, after the addition, the temperature was raised to 100°C, and 100g (0.61mol) of 2-bromocyclopentanone was added dropwise. Hours, the reaction was completed, and vacuum distillation was performed to obtain 200 g of a DMF solution containing 2-cyclopentenone (compound of formula I) (the molar yield was 80%, and the mass concentration was 20%) for use.
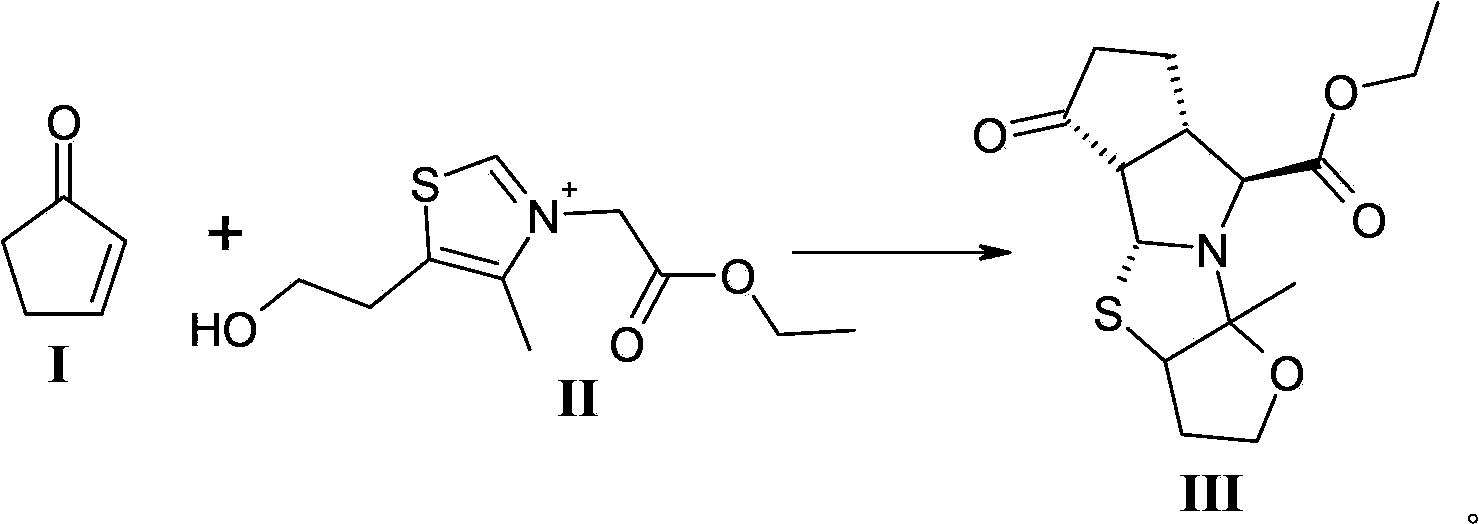
[0026] Dissolve 50.0g (0.16mol) of the compound of formula II in 50mL of water to prepare an aqueous solution; control the temperature of the reaction system at -10 to 10°C, add 84mL of the above-mentioned DMF solution containing 2-cyclopentenone (0.24mol) dropwise to the formula In the aqueous solution of compound II; after dropping, keep warm and stir at -10-10°C for 5-10 minutes; slowly add 21.1g (0.21mol) Et 3 N; After dropping, continue to keep warm and react at -10-10°C for 2-3 hours; naturally rise to room temperature and reac...
Embodiment 2
[0028] Add 300mL N-methylacetamide, 101g (1.0mol) triethylamine, 2.1g (0.05mol) LiCl, 0.1g (0.0015mol) hydroquinone into a 500mL three-necked flask, raise the temperature to 160°C after the addition, and add dropwise 100g (0.84mol) 2-chlorocyclopentanone, dropwise, heat preservation reaction for 3 to 4 hours, after the reaction is completed, vacuum distillation to obtain N-methyl ethyl alcohol containing 2-cyclopentenone (compound of formula I). Amide solution 374g (molar yield is 70%, mass concentration is 13%), stand-by;
[0029] 50.0g (0.16mol) of the compound of formula II was dissolved in 50mL of water to prepare an aqueous solution; the temperature of the reaction system was controlled at -10 to 10°C, and 159mL of the above-mentioned N-methylacetamide containing 2-cyclopentenone (0.24mol) Add the solution dropwise to the aqueous solution of the compound of formula II; after dropping, keep warm and stir at -10~10°C for 5~10 minutes; slowly add 21.1g (0.21mol) Et 3 N; Aft...
Embodiment 3
[0031] Weigh 17g (0.21mol) of 2-cyclopentenone and dissolve it in 94g of DMF to prepare 117mL of 2-cyclopentenone-containing DMF solution with a mass concentration of 18%.
[0032] 50.0g (0.16mol) of the compound of formula II was dissolved in 50mL of water to prepare an aqueous solution; the temperature of the reaction system was controlled at -10 to 10°C, and 117mL of the above-mentioned DMF solution containing 2-cyclopentenone (0.21mol) was added dropwise to the formula In the aqueous solution of compound II; after dropping, keep warm and stir at -10-10°C for 5-10 minutes; slowly add 21.1g (0.21mol) Et 3N; After dropping, continue to keep warm and react at -10-10°C for 2-3 hours; naturally rise to room temperature and react for 13-15 hours, then cool down in an ice bath to -10-10°C and solids will precipitate, stir for 0.5 hours After that, the solid was collected by suction filtration and dried to obtain 40 g of the compound of formula III (molar yield: 80.0%, HPLC purity:...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com