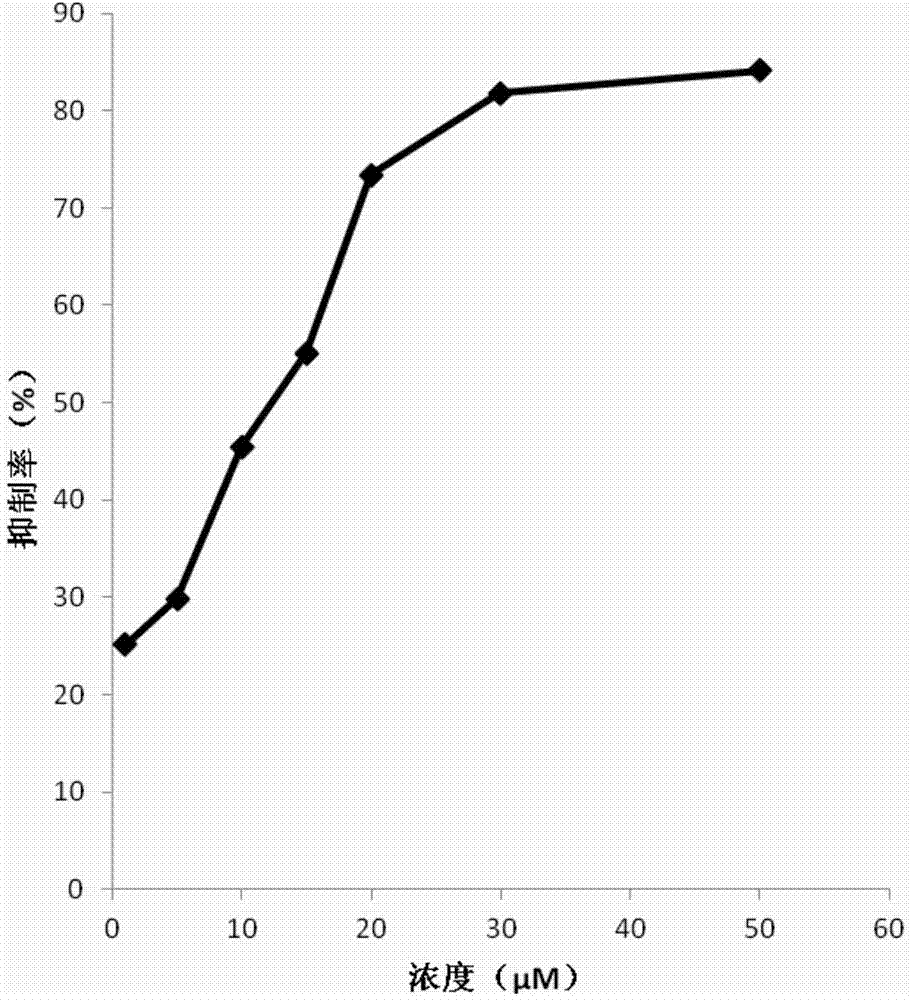
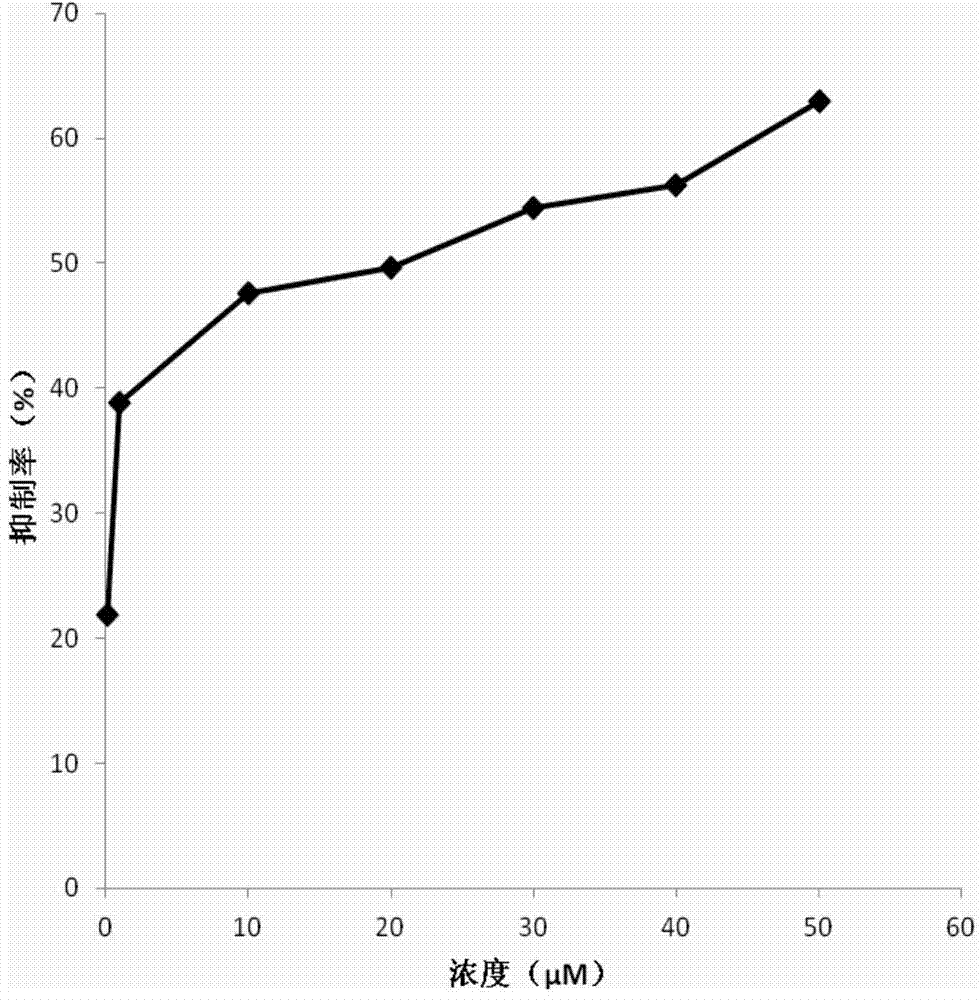
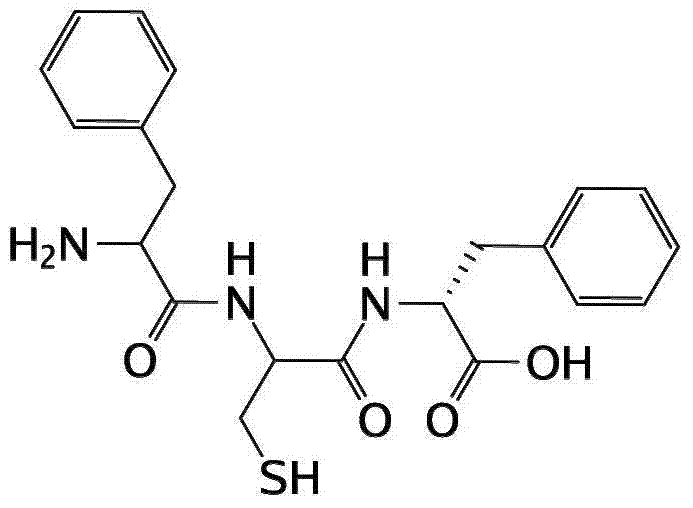
New Delhi metallo-beta-lactamase inhibitory peptide and application thereof
A technology of lactams, phe-cys-d-phe, applied in the treatment of drug-resistant bacterial infections, tripeptide inhibitors of metallo-β-lactamases, preparation of antibacterial drugs and prevention, can solve difficult problems in clinical treatment , to achieve the effect of easy control of product quality, simple structure and simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Preparation of Phe-Cys-D-Phe
[0067] 1. Weigh 2.0g of MBHA (4-toluene hydrogen amine) resin into a clean and dry reaction tube, add an appropriate amount of DMF (dimethylformamide), activate for about 30 minutes, and then weigh Fmoc (fluorenylmethyloxycarbonyl) protection Add 1mmol of Phe, 150mg of DMAP (4-dimethylaminopyridine), add 1ml of 98% DIC (N,N′-diisopropylcarbodiimide) to the reaction tube, and react with DMF as the solvent for 3h. After the reaction is completed, wash with DMF for 4 to 6 times, add appropriate amount of pyridine and acetic anhydride, the volume ratio is 1:1, and react for 30 minutes. After the reaction, wash with DMF 4 to 6 times. Then use 20% piperidine solution to remove the Fmoc of amino acid, remove twice for a total of 15min, 10min+5min. Then wash 4 times with DMF, wash 2 times with methanol, take out a small amount of resin and detect it with ninhydrin detection reagent, the detection is blue, and the next step reaction can be carrie...
Embodiment 2
[0072] Preparation of Antibacterial Pharmaceutical Composition Containing Phe-Cys-D-Phe Inhibitor Peptide (Capsule)
[0073]
[0074] According to the amount of 100 capsules, weigh the above auxiliary materials, grind and sieve them, and mix them evenly, then add cefuroxime sodium and Phe-Cys-D-Phe by equal addition method, fully grind to make them disperse evenly, and then Pass through a 80-mesh sieve, and then fill into capsules.
PUM
| Property | Measurement | Unit |
|---|---|---|
| control rate | aaaaa | aaaaa |
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com