Method for preparing telaprevir intermediate
A compound and selected technology, applied in the field of medicine and chemical industry, can solve the problems of harsh process conditions, high production costs, and unavailable oxalate raw materials, and achieve the effects of easy industrialization, mild process conditions, and avoiding chiral raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
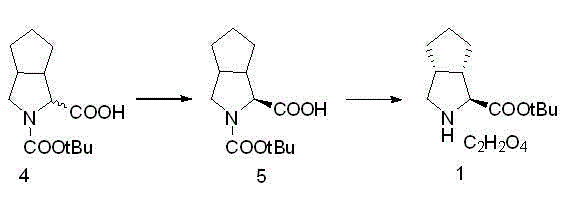
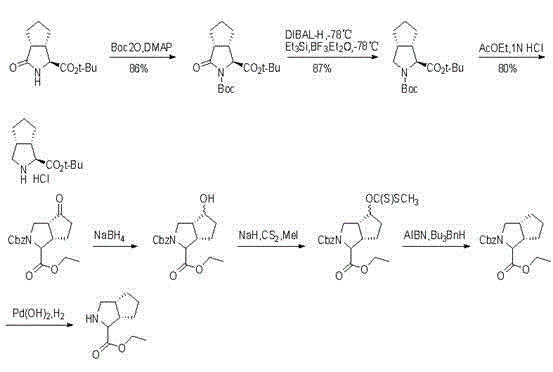
Embodiment 1
[0057] 3-Azabicyclo[3,3,0]oct-2-ene (compound 2):
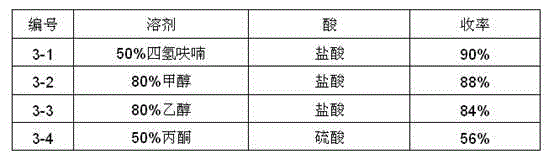
[0058] Add 14.76g (100mmol) of 3-azabicyclo[3.3.0]octane hydrochloride and 60ml of water into the reaction flask, stir to dissolve, cool down to -5~5°C, add 10.5g (105mmol) of 12% sodium hypochlorite dropwise , After the addition, keep the reaction for 4 hours, TLC monitors the complete conversion of the reactant to the product (developing solvent: ethyl acetate: n-hexane = 1:3). After the reaction is complete, add 50 mL of isopropyl ether and stir, separate the organic layer, add 30 mL of isopropyl ether to the water layer to extract twice, combine the organic layers, wash with water until neutral, dry with 20 g of anhydrous sodium sulfate, filter off the desiccant, and concentrate under reduced pressure Until there is no slip-out, 10.2 g of oily substance is obtained. Dissolve 4.7g of potassium hydroxide in 47ml of 95% ethanol (volume ratio), raise the temperature to about 80°C, dissolve the above oil in 102ml of 95% ethan...
Embodiment 2
[0069] 3-Azabicyclo[3,3,0]oct-2-ene (compound 2):
[0070] Add 14.76g (100mmol) of 3-azabicyclo[3.3.0]octane hydrochloride and 100ml of acetone into the reaction flask, stir to dissolve, cool down to -5~5℃, add N-chlorosuccinimide dropwise 14g (105mmol), keep the reaction for 2h after the addition, and monitor the complete conversion of the reactant to the product by TLC (developing solvent: ethyl acetate: n-hexane = 1:3). After the reaction is complete, spin off the acetone, add 50mL of isopropyl ether and 50ml of water, stir, separate the organic layer, add 30ml of isopropyl ether to the water layer to extract twice, combine the organic layers, wash with water until neutral, dry with 20g of anhydrous sodium sulfate, The desiccant was filtered off, and concentrated under reduced pressure until no slip-out material was obtained to obtain 10.2 g of an oily substance. Dissolve 3.4g of sodium hydroxide in 40ml of 90% methanol (volume ratio), heat up to about 60°C, dissolve the a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com