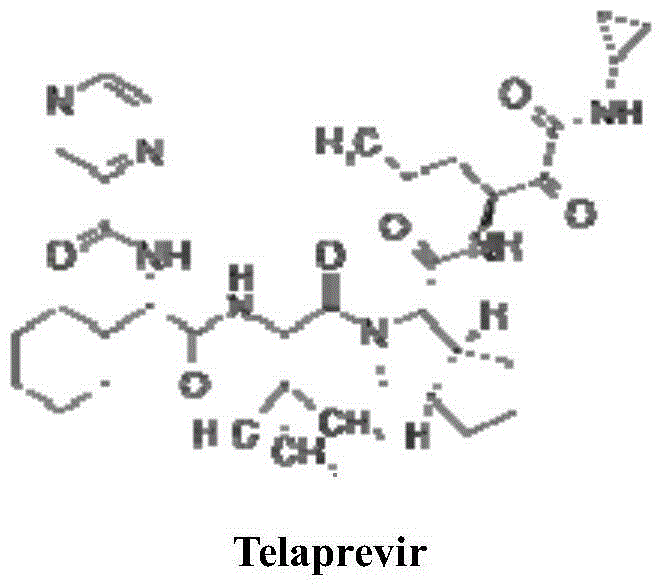
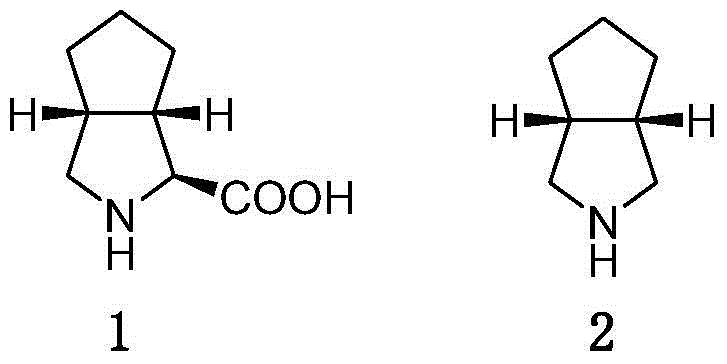Telaprevir synthesis intermediate and preparation method thereof
A compound and selected technology, applied in the direction of organic chemistry, can solve the problems of cumbersome operation and expensive preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030]
[0031] Add 4g (13.4mmol) racemic-2-(benzyloxycarbonyl)-4-(carbonyl) octahydrocyclopentadieno[c]pyrrole-1-carboxylic acid ethyl ester in the four-necked flask, 1.36g ( 14.7mmol) 1,2-ethanedithiol, 3.8g (26.8mmol) boron trifluoride ether, 40ml dichloromethane, stirred at room temperature for 30min to stop the reaction, washed twice with saturated sodium bicarbonate solution (40ml*2) , washed once with water (40ml*1), dried the organic phase with anhydrous magnesium sulfate, filtered with suction, and spin-dried. Obtain 4g of target compound (yield 96%), MS (m / z): 407.12[M+H] + ; 1 HNMR (CDCl 3 .400MHz) δ: 1.1-1.2(t,3H),1.65-1.68(m,1H),2.0-2.21(m,2H),2.21-2.34(m,2H),2.85-2.852(m,1H), 3.0-3.05(m,1H),3.27-3.276(m,4H),3.5-3.8(m,2H),4.0-4.2(m,2H),5.0-5.1(m,2H),7.2-7.4(m ,5H).
Embodiment 2
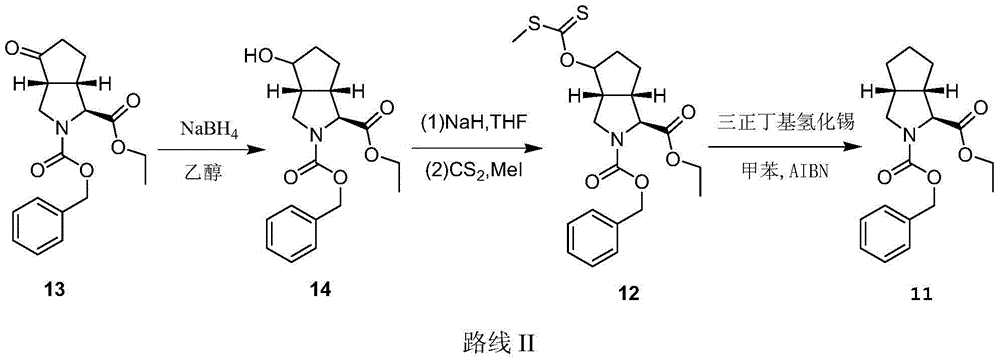
[0033]
[0034] Add 7.62g (18.7mmol) compound 12, 76.2g (10 times mass fraction) Raney nickel, 700mL tert-butanol, nitrogen protection, reflux reaction for 8h to stop the reaction, suction filtration, and spin dry to obtain 3.8g target Compound (65% yield), MS(m / z): 317.16[M+H] + .
Embodiment 3
[0038] Add 12.18g (36.6mmol) of compound 13 and 120mL of ethanol into the four-neck flask, stir to dissolve it, cool the temperature to 0°C in an ice-salt bath, add 1.44g of sodium borohydride in batches, slowly rise to room temperature after adding, and continue stirring After 30 minutes, 4.5ml of acetic acid was added to terminate the reaction, the ethanol was spin-dried, and 350ml of ethyl acetate was added, washed with saturated ammonium chloride solution and saturated sodium bicarbonate solution respectively, dried, and spin-dried. 11.5 g of compound 14 was obtained (95% yield), MS (m / z): 333.14 [M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com