Preparation method of telaprevir intermediate and salt thereof
A cyclohexyl glycinate, pyrazine technology, applied in the direction of organic chemistry and the like, can solve the problems of oxidative side reactions, cumbersome synthesis routes, easy to produce carcinogens, etc., and achieves low price, simple post-processing and reduced production costs. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
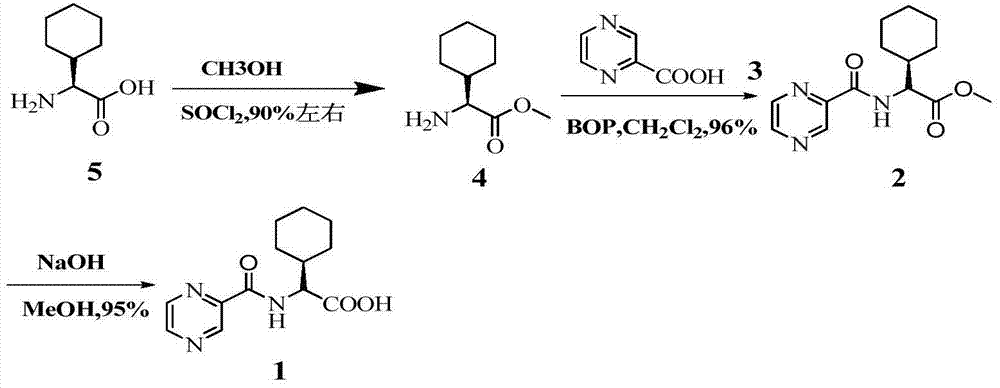
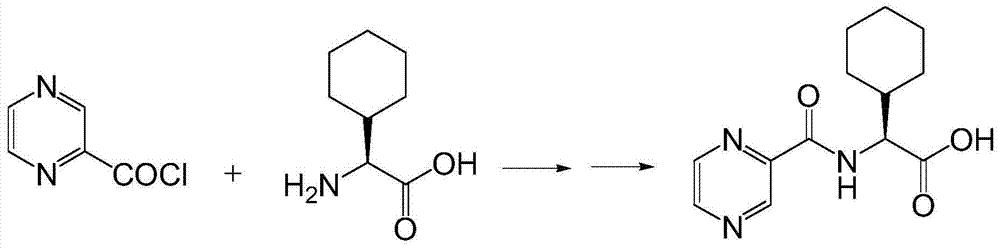
[0033] At room temperature, thionyl chloride (29mL, 0.4mol) was added dropwise to pyrazine-2-carboxylic acid (5g, 0.04mol), 1 drop of DMF was added as a catalyst, and the reaction was refluxed at 78°C for 3h. Concentrate under reduced pressure and dissolve with 1,4-dioxane to prepare a pyrazine-2-formyl chloride solution for use.
Embodiment 2
[0035] At room temperature, thionyl chloride (14.5mL, 0.2mol) was added dropwise to pyrazine-2-carboxylic acid (5g, 0.04mol), 1 drop of DMF was added as a catalyst, and the reaction was refluxed at 78°C for 3h. Concentrate under reduced pressure and dissolve with 1,4-dioxane to prepare a pyrazine-2-formyl chloride solution for use.
Embodiment 3
[0037] At room temperature, oxalyl chloride (50.8g, 0.4mol) was slowly added dropwise to pyrazine-2-carboxylic acid (5g, 0.04mol) in 50mL dichloromethane solution, and 1 drop of DMF was added dropwise to catalyze. After the addition, the temperature was slowly raised to room temperature, stirred overnight, dried over anhydrous magnesium sulfate, and spin-dried to obtain pyrazine-2-formyl chloride. Dissolve in 1,4-dioxane solution for use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com