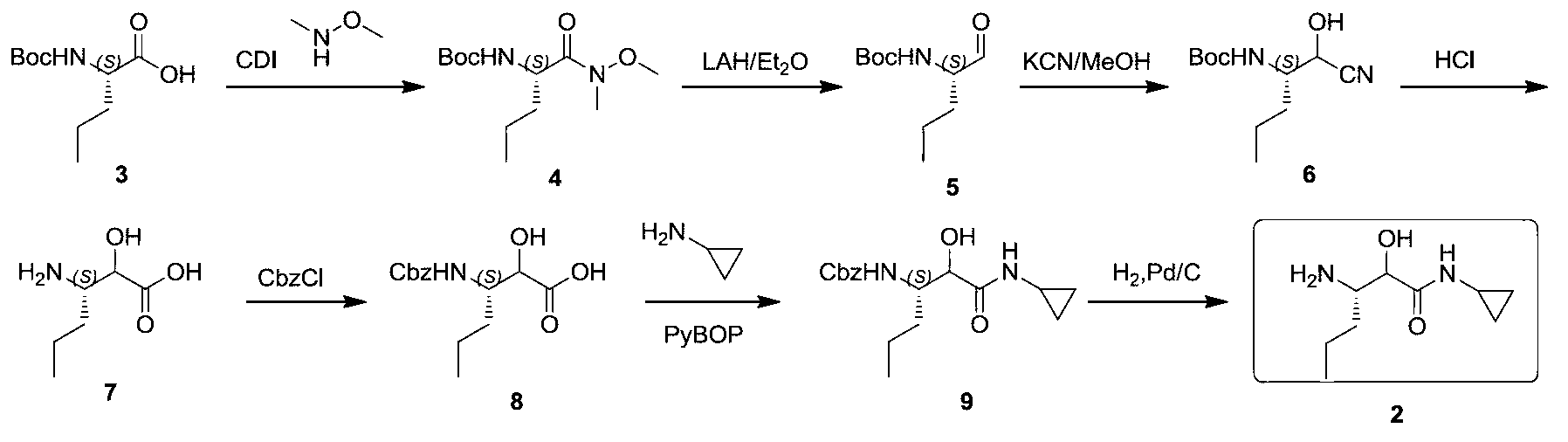
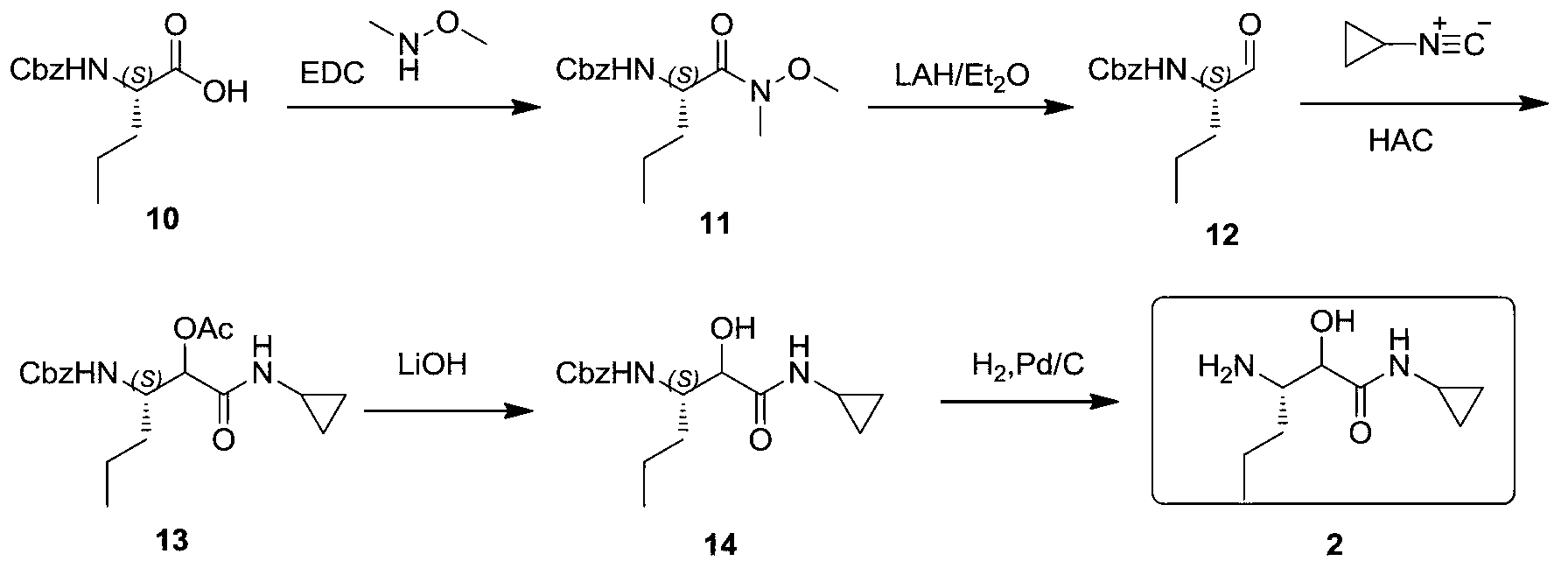
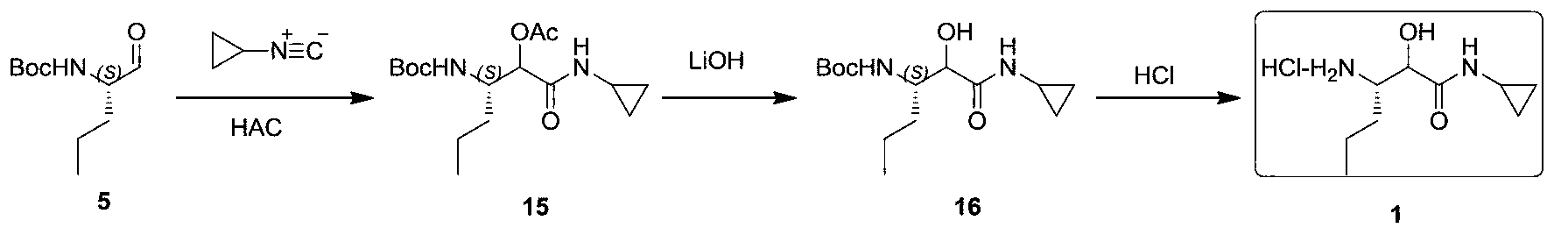
Synthetic method of (3S)-3-amino-N-cyclopropyl-2-hydroxyhexanamide hydrochloride
The technology of a hydroxycaproamide and a synthesis method is applied in the preparation of carboxylic acid amides, chemical instruments and methods, preparation of organic compounds, etc., and can solve the problems of chirality in molecular structure, complex synthesis method, and high economic cost, and achieve raw materials. Easy to obtain, simple to operate, and the effect of short synthesis cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 , Synthesis of (3S)-3-(benzyl((S)-1-phenylethyl)amino)-2-hydroxyl-4-hexenoic acid tert-butyl ester
[0033] Add n-butyllithium (68.7mL×1.6M, 0.11mol) dropwise into a solution of (S)-N-benzyl-1-phenethylamine (23.2g, 0.11mol) in tetrahydrofuran (300mL) and control the external temperature at - 50~-78°C. Then, a solution of tert-butyl sorbate (16.8g, 0.1mol) in tetrahydrofuran (100mL) was added to the reaction solution, and reacted at -50~-78°C for two hours, and then camphorsulfonazine (25.2g, 0.11mol), and reacted at -50~-78°C for one hour, naturally warmed to room temperature around 25°C overnight, added saturated ammonium chloride (300mL) to quench the reaction, and extracted with methyl tert-butyl ether (500mL) , and washed with saturated brine (300mL), the organic phase was concentrated to dryness under reduced pressure to obtain (3S)-3-(benzyl((S)-1-phenylethyl)amino)-2-hydroxy-4-hexenoic acid Tert-butyl ester (24.1g), yield 61%.
Embodiment 2
[0034] Example 2 , Synthesis of (3S)-3-(benzyl((S)-1-phenylethyl)amino)-2-hydroxyl-4-hexenoic acid
[0035] (3S)-3-(Benzyl((S)-1-phenylethyl)amino)-2-hydroxy-4-hexenoic acid tert-butyl ester (24.1 g, 61 mmol) was added to 98% formic acid (60 mL) , stirred at 20-30°C for 12 hours, the reaction solution was concentrated to dryness under reduced pressure, methyl tert-butyl ether (100mL) was added, and the precipitated product was filtered to obtain (3S)-3-(benzyl((S)-1-benzene Ethyl)amino)-2-hydroxy-4-hexenoic acid (19.7g), yield 96%.
Embodiment 3
[0036] Example 3 , Synthesis of (3S)-3-(benzyl((S)-1-phenylethyl)amino)-N-cyclopropyl-2-hydroxyl-4-hexenamide
[0037] (3S)-3-(benzyl((S)-1-phenylethyl)amino)-2-hydroxy-4-hexenoic acid (19.7g, 58mmol), N-hydroxysuccinimide (13.3g , 116mmol) into dimethylformamide (100mL), stirred at 20-30°C for 0.5 hour, added 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC. HCl) condensing agent (22.2g, 116mmol), stirred at 20-30°C for 1 hour, added cyclopropylamine (6.6g, 116mmol), stirred at 20-30°C for 12 hours, added water (200mL) and ethyl acetate (300mL ), the organic layer was washed once with saturated sodium bicarbonate (200mL), concentrated to dryness, added methyl tert-butyl ether (100mL), and the precipitated product was filtered to obtain (3S)-3-(benzyl((S)-1-benzene Ethyl)amino)-N-cyclopropyl-2-hydroxy-4-hexenamide (18.9g), yield 86%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com