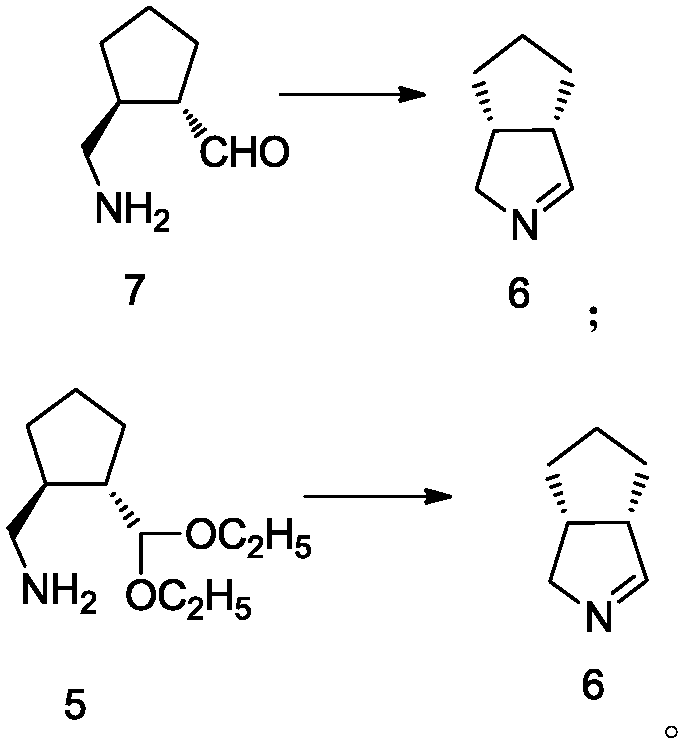
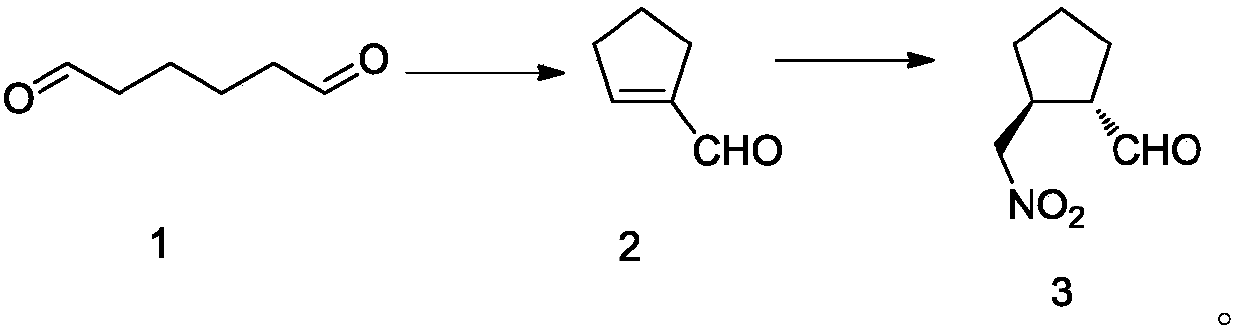
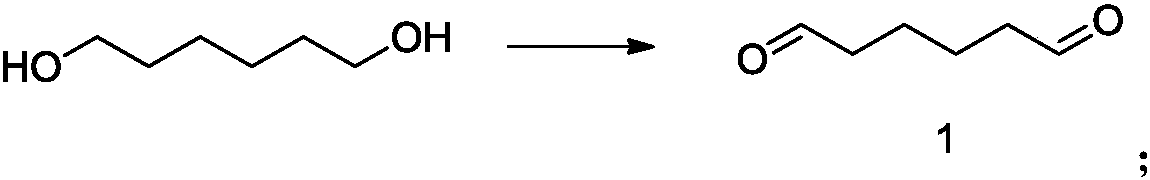
Intermediate of telaprevir and preparation method thereof
A compound and solvent technology, applied in the preparation of telaprevir intermediates and the field of intermediates, can solve the problems of high cost, low efficiency, difficult industrialized production and the like, and achieve the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0131] In a 500mL three-necked flask, add 118g (1mol) hexanediol, 1200mL dichloromethane and 900mL water, cool the reaction to 0-5°C in an ice-water bath, add 5.9g (50mmol) KBr, 0.16g (1mmol) TEMPO, 35.8g successively (100mmol) Na2HPO4.12H2O, 15.6mg (100mmol) NaH2PO4.2H2O, control the reaction temperature 0-5 ℃, slowly dropwise add 1360mL (2mol) 7.5% NaClO solution, while keeping the reaction temperature below 5 ℃. After 2 hours of reaction, the starting material basically disappeared. The reaction was stopped, the organic phase was directly separated, the aqueous layer was extracted with 400 mL of DCM, the organic phases were combined, washed once with 600 mL of saturated sodium bicarbonate solution, once with 600 mL of water, and dried over anhydrous sodium sulfate. After filtration, the filtrate was concentrated under reduced pressure to obtain 105 g of a brown transparent liquid, namely compound 1, with a yield of 92%.
Embodiment 2
[0133]Add 118g (1mol) hexanediol, 1200mL dichloromethane and 900mL water to a 500mL three-neck flask, cool the reaction to 0-5°C in an ice-water bath, add 5.9g (50mmol) KBr, 0.16g (1mmol) TEMPO, 35.8g (100mmol) Na2HPO4.12H2O, 15.6mg (100mmol) NaH2PO4.2H2O, control reaction temperature 0-5 ℃, slowly drop 1360mL (2mol) 7.5% NaClO solution, keep reaction temperature below 5 ℃ simultaneously. After 2 hours of reaction, the starting material basically disappeared. Stop the reaction, separate the organic phase directly, extract the aqueous layer with 400 mL of 2-dichloroethane, combine the organic phases, wash once with 600 mL of saturated sodium bicarbonate solution, once with 600 mL of water, and dry over anhydrous sodium sulfate. After filtration, the filtrate was concentrated under reduced pressure to obtain 100 g of a brown transparent liquid, namely compound 1, with a yield of 88%.
Embodiment 3
[0135] Add 118g (1mol) hexanediol, 1200mL dichloromethane and 900mL water to a 500mL three-necked flask, cool the reaction to 0-5°C in an ice-water bath, add 5.9g (50mmol) KBr, 0.16g (1mmol) TEMPO, 41g carbonic acid Sodium hydrogen (2.85mol) was used to control the reaction temperature at 0-5°C, and 1360mL (2mol) of 7.5% NaClO solution was slowly added dropwise while keeping the reaction temperature below 5°C. After 2 hours of reaction, the starting material basically disappeared. Stop the reaction, separate the organic phase directly, extract the aqueous layer with 400mL DCM, combine the organic phases, wash once with 600mL saturated sodium bicarbonate solution, once with 600mL water, and dry over anhydrous sodium sulfate. After filtration, the filtrate was concentrated under reduced pressure to obtain 62 g of a brown transparent liquid, namely compound 1, with a yield of 54%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com