Pegylated interferon injection and preparation method thereof
A technology of polyethylene glycol and interferon, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve problems such as infectious blood diseases, protein molecular changes, and easy oxidation. Achieve the effects of improving safety and stability, reducing manufacturing costs, and simplifying the process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
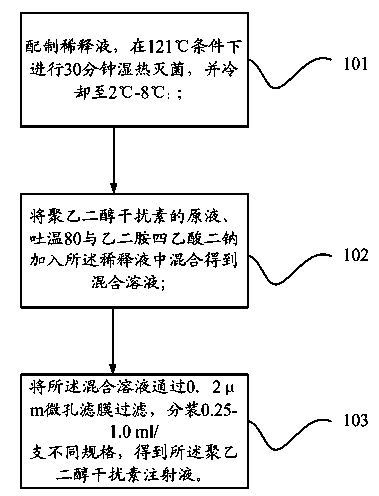
[0025] The present invention also provides a preparation method of pegylated interferon injection, such as figure 1 As shown, it includes the following steps:
[0026] Step 101: Prepare a dilution solution, perform moist heat sterilization at 121°C for 30 minutes, and cool to 2°C-8°C;
[0027] Step 102: adding the stock solution of pegylated interferon, Tween 80 and disodium edetate to the diluent and mixing to obtain a mixed solution;
[0028] Step 103: Filter the mixed solution through a 0.2 μm microporous membrane, and pack into different specifications of 0.25-1.0 ml / bottle to obtain the pegylated interferon injection.
[0029] In another preferred embodiment of the present invention, the content of the pegylated interferon is 180 μg / ml, and the content of the Tween 80 is 0.1-0.3 mg / ml. The content of disodium edetate is 1-2 mg / ml; the pegylated interferon injection also includes phosphate buffer, and the content of the phosphate buffer is 0.1M-0.3M.
[0030] Further, w...
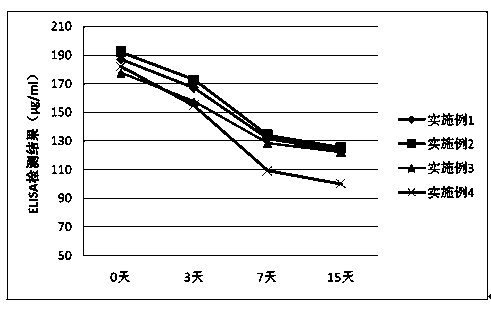
Embodiment 1
[0033] The components of pegylated interferon α2b injection are: pegylated interferon α2b 180mg, Tween 80 100mg, disodium edetate 1g, disodium hydrogen phosphate 12.68g, sodium dihydrogen phosphate 3.43g , 4.89 g of sodium chloride, an appropriate amount of water for injection, and the total volume of the above components is 1000 ml.
[0034] The preparation method of pegylated interferon injection comprises the following steps:
[0035] Weigh 12.68 g of disodium hydrogen phosphate, 3.43 g of sodium dihydrogen phosphate, and 4.89 g of sodium chloride, dissolve them with an appropriate amount of water for injection, add them to the above solution, and set the volume to the specified volume. The pH value is between 6.5-7.5. Then perform moist heat sterilization at 121°C for 30 minutes, and cool to 2°C-8°C to obtain a dilution;
[0036] Prepare 1% Tween 80 solution: weigh 100 mg of Tween 80, add appropriate amount of water for injection, and stir until the solution is evenly mix...
Embodiment 2
[0040]The components of peginterferon α2b injection are: peginterferon α2b 180mg, Tween 80 200mg, disodium edetate 1g, disodium hydrogen phosphate 12.68g, sodium dihydrogen phosphate 3.43g , 4.89 g of sodium chloride, an appropriate amount of water for injection, and the total volume of the above components is 1000 ml.
[0041] The preparation method of pegylated interferon injection comprises the following steps:
[0042] Weigh 12.68 g of disodium hydrogen phosphate, 3.43 g of sodium dihydrogen phosphate, and 4.89 g of sodium chloride, dissolve them with an appropriate amount of water for injection, add them to the above solution, and set the volume to the specified volume. The pH value is between 6.5-7.5. Then perform moist heat sterilization at 121°C for 30 minutes, and cool to 2°C-8°C to obtain a dilution;
[0043] Prepare 1% Tween 80 solution: weigh 200 mg of Tween 80, add appropriate amount of water for injection, and stir until the solution is evenly mixed;
[0044] T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com