Recombinant bovine long-term interferon and fusion protein for preparing same and preparation method of recombinant bovine long-term interferon
A technology of fusion protein and bovine interferon, applied in the field of biological genetic engineering, can solve the problems of small molecular weight of interferon, unfavorable application, short half-life, etc., and achieve the effects of avoiding denaturation and renaturation, improving half-life and shortening expression time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] A fusion protein consisting of bovine interleukin 2 and bovine interferon α, the preparation method of which is as follows:
[0073] 1. Acquisition and amplification of bovine interleukin 2 (IL-2) and bovine interferon α (IFN-α) target genes
[0074] Primer design:
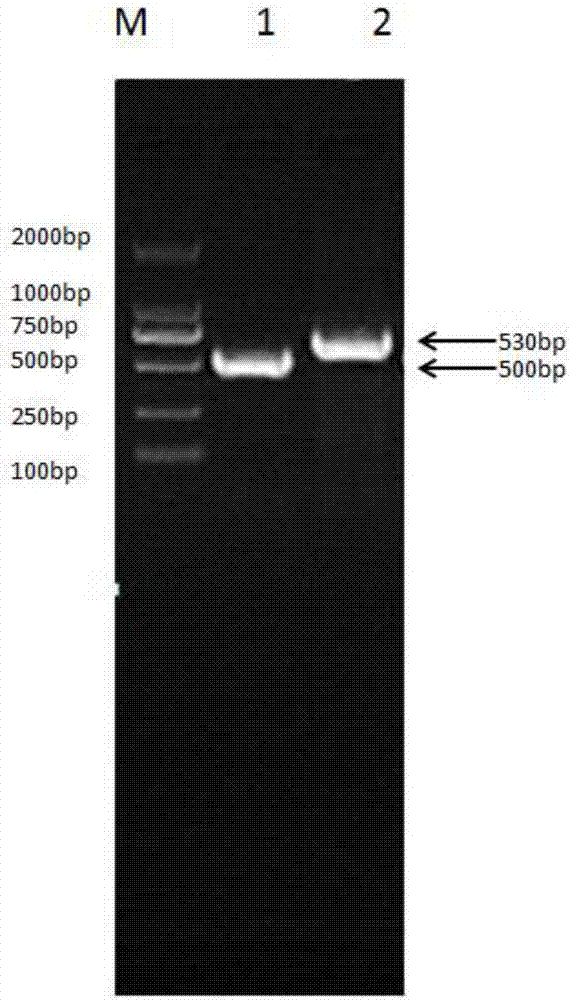
[0075] According to the target gene sequence reported in Genebank, the synthetic primers were designed and synthesized as shown in Table 1. The EcoRI restriction site and Linker sequence were respectively introduced into the upstream primer and downstream primer of bovine interleukin 2, and the upstream primer and downstream primer of bovine interferon alpha Linker sequence and XhoI restriction site were introduced respectively.
[0076] Table 1 PCR amplification primers
[0077]
[0078] RT-PCR to obtain the target gene:
[0079] RNA was extracted from bovine liver tissue, and the target genes of IL-2 and IFN-α were obtained by reverse transcription, and the gene sequences of the two were shown in SE...
Embodiment 2
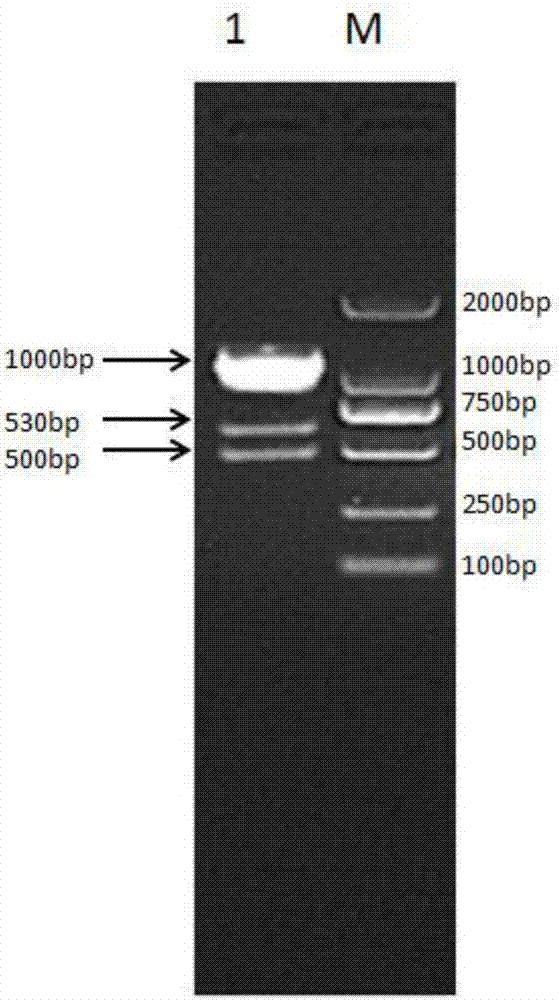
[0112] A fusion protein composed of bovine interleukin 2 and bovine interferon α, the others are the same as in Example 1, except that the Escherichia coli BL21 (DE3) competent cells are replaced by BL21 (DE3) competent cells with pGro7 plasmid cell. The SDS-PAGE electrophoresis inspection result of its fusion protein is compared with embodiment 1, and the dominant expression band at about 55.9KD in the supernatant is thicker, indicating that after introducing molecular chaperone pGro7, the expression of the target protein in the supernatant is more Well, higher amounts of fusion protein are obtained. Most of the proteins expressed in E. coli exist in inclusion bodies; by introducing molecular chaperones into the expression strains, the co-expressed proteins can be correctly folded to achieve protein soluble expression.
[0113] The BL21(DE3) competent cells carrying the pGro7 plasmid were purchased from Shanghai Inshore Science & Technology Co., Ltd. / Simbano Biotech, Cat. No...
Embodiment 3
[0115] A fusion protein consisting of bovine interleukin 2 and bovine interferon α, the preparation method of which is as follows:
[0116] 1. Acquisition and amplification of bovine interleukin 2 (IL-2) and bovine interferon α (IFN-α) target genes
[0117] IL-2 and IFN-α in Example 1 are optimized, and IL-2 and IFN-α target genes are artificially synthesized. After optimization, the nucleotide sequences of the two are as SEQUENCE LISTING 400 and SEQUENCE LISTING 400 respectively As shown in .
[0118] 1.1 Codon optimization
[0119] There are 64 genetic codes, but most organisms tend to use a subset of these. Those that are most frequently used are called optimal codons, and those that are not frequently used are called rare or low-usage codons. Virtually every organism commonly used for protein expression or production (including E. coli, yeast, mammalian cells, plant cells, and insect cells) exhibits some degree of difference or bias in codon usage. The expression effic...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com